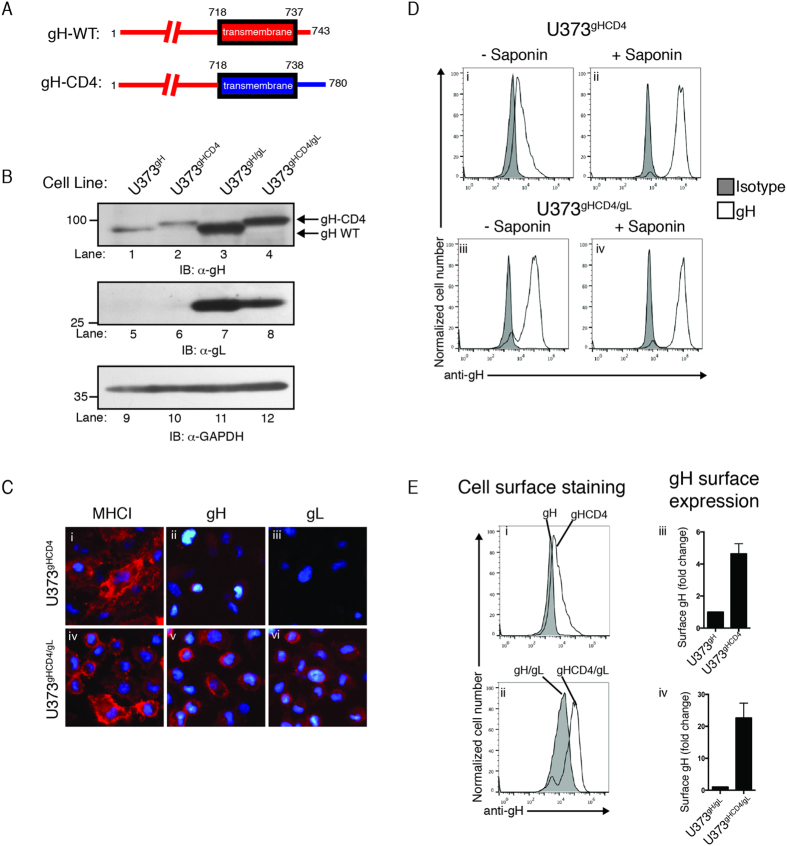
Figure 5. The chimeric gH-CD4 possesses enhanced trafficking properties.
(A) A schematic representing the luminal and transmembrane domains and the cytoplasmic tail of wild type gH and the gH-CD4 chimeric protein. (B) U373gH, U373gHCD4, U373gH/gL, and U373gHCD4/gL cells were subjected to immunoblot analysis for gH (lanes 1–4), gL (lanes 5–8), and GAPDH (lanes 9–12). Molecular weight standards and proteins are indicated. (C) U373gHCD4 and U373gHCD4/gL cells were subjected to analysis by fluorescence microscopy for properly-folded MHC class I (i and iv), gH (ii and v), or gL (iii and vi) using the respective antibodies followed by incubation with an IgG-specific Alexa-555 fluorescent antibody. Hoechst reagent allowed visualization of the nucleus. (D) U373gHCD4 and U373gHCD4/gL cell lines were analyzed by flow cytometry for gH expression either without (i and iii), or with (ii and iv) cell permeabilization with saponin treatment. Background fluorescence levels were established by staining with an IgG isotype (gray peaks). (E) The amount of cell surface gH versus internalized gH in U373gH and U373gH/gL cells was compared to U373gHCD4 (i) and U373gHCD4/gL (ii) cells by flow cytometry. Expression levels were calculated as percent of total gH expressed on the cell surface, and displayed as fold change compared to U373gH(iii) and U373gH/gL cells (iv).