Abstract
Environmental stressors induce coping strategies in the majority of individuals. The stress response, involving the activation of the hypothalamic-pituitary-adrenocortical axis and the consequent release of corticosteroid hormones, is indeed aimed at promoting metabolic, functional, and behavioral adaptations. However, behavioral stress is also associated with fast and long-lasting neurochemical, structural, and behavioral changes, leading to long-term remodeling of glutamate transmission, and increased susceptibility to neuropsychiatric disorders. Of note, early-life events, both in utero and during the early postnatal life, trigger reprogramming of the stress response, which is often associated with loss of stress resilience and ensuing neurobehavioral (mal)adaptations. Indeed, adverse experiences in early life are known to induce long-term stress-related neuropsychiatric disorders in vulnerable individuals. Here, we discuss recent findings about stress remodeling of excitatory neurotransmission and brain morphology in animal models of behavioral stress. These changes are likely driven by epigenetic factors that lie at the core of the stress-response reprogramming in individuals with a history of perinatal stress. We propose that reprogramming mechanisms may underlie the reorganization of excitatory neurotransmission in the short- and long-term response to stressful stimuli.
1. Introduction
Life experiences often produce uncertainty or threat and trigger a physiological response, the so-called “stress response,” aimed at promoting adaptation and improving survival [1]. The stress response, including fast and transient activation of the autonomic nervous system and of the hypothalamic-pituitary-adrenocortical (HPA) axis, implies release of catecholamines and corticosteroids (mainly cortisol in humans and corticosterone in rodents). Corticosteroids exert their function through the activation of the high-affinity mineralocorticoid receptor (MR) and the low-affinity glucocorticoid receptor (GR), which are widely expressed both at peripheral level and in the brain. Corticosteroids, together with regulating metabolism, food intake, and the immune system, modulate brain function, neuronal transmission, and plasticity, especially in corticolimbic areas [2, 3].
In the high majority of individuals, the stress response is able to activate coping strategies to adverse environmental changes, promoting stress resilience. However, in vulnerable subjects, the stress response may become dysregulated and induce maladaptive changes, which in turn underlie increased susceptibility to stress-related neuropsychiatric diseases [4–6]. Daskalakis and collaborators [7] proposed the 3-hit concept (hit 1: genetic predisposition, hit 2: early-life environment, and hit 3: later-life environment) to explain why some individuals can cope with adverse events and remain resilient while others are vulnerable and succumb to stress-related disorders. This concept readapts the cumulative hypothesis of stress [8], which indicates that accumulating failures to cope with stressors lead to dramatic consequences on the individuals, consistent with increased vulnerability to psychiatric disorders [1]. Of note, the 3 hits also endorse the mismatch hypothesis of psychiatric disorders, suggesting that early-life adversities can prepare the individuals to cope with future life similar challenges; conversely, the coping strategies are compromised when the later-life events exhibit mismatch with the early-life environment [9, 10].
In this context, during the last decades, there have been a growing number of studies on short- and long-term consequences of early-life stress (including manipulations during the prenatal period and/or the early phase of postnatal development), suggesting an increased interest in the impact of early-life adversities on stress response and susceptibility to neuropsychiatric disorders in the adulthood [11–24].
In the first sections of the present review, we will summarize functional, morphological, and epigenetic changes in adults induced by stress exposure during adulthood, or during early life. Finally, in order to recapitulate the interplay between life adversities at early stages and in the adulthood, we will introduce the concept of “reprogramming,” a process whereby a stimulus or insult, during a sensitive period of development, has lasting and/or lifelong significance, inducing readaptation of the stress response.
2. Acute and Chronic Stress at Synapses: Corticosterone-Dependent Effects of the Stress Response
A growing body of literature has analyzed the multifaceted effects of different stress protocols and of corticosteroids (mainly corticosterone) on neurotransmission, neuronal plasticity, and behavior (see below). MR and GR are nuclear receptors, acting as transcription factors, ultimately leading to regulation of gene expression. However, more recently, compelling evidence has reported fast effects of corticosteroids on neuronal excitability, in line with early nongenomic mechanisms that are likely dependent on membrane-located receptors (for recent reviews, see [25–27]).
In the next sections, we will review the most recent findings on fast and delayed effects of acute and repeated stress, mediated by genomic and nongenomic action of corticosteroids in the brain.
2.1. Corticosterone-Dependent Effects of Stress on Excitatory Neurotransmission
A number of studies have been performed to unravel the time-dependent and brain area-specific effects of stress on neuronal excitability and cognitive processes (for recent reviews, see [2, 26–28]). The changes in neuronal excitability and synaptic plasticity induced by stress are the result of an imbalance of excitatory (glutamatergic) and inhibitory (GABAergic) transmission, leading to long-lasting (mal)adaptive functional modifications [28–34]. Although both glutamate and GABA transmission are critically associated with stress-induced alteration of neuronal excitability [32, 34], the present review will focus on the modulation of glutamate release and transmission induced by stress and glucocorticoids.
Genomic and nongenomic effects of acute stress were characterized in both the hippocampus and the prefrontal cortex. Acute stress was consistently reported to rapidly enhance the frequency of miniature excitatory currents (mEPSCs) at hippocampal synapses, thus suggesting increased probability of glutamate release, through nongenomic action of corticosterone, and activation of membrane-located pre- and postsynaptic MR [29, 35–37]. On the other hand, slower genomic effects of acute stress in the hippocampus are mainly mediated by GR, which prevents synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor potentiation and enhances voltage-dependent calcium currents and mEPSCs amplitude. This leads to steady depolarization and attenuation of firing activity [29, 38, 39] and impaired long-term potentiation (LTP) [30, 40].
Partially different effects of acute stress were reported in the prefrontal cortex, where nongenomic mechanisms, despite being involved in the priming of excitatory synapses, are not sufficient to induce changes in glutamate release and transmission [41–43]. Indeed, it was found that corticosterone rise induced by acute footshock stress increases the size of the readily releasable pool of glutamatergic vesicles in the prefrontal cortex, through completely nongenomic mechanisms involving both MR and GR located at synaptic sites. However, in the same brain area, slower genomic mechanisms are required to enhance presynaptic glutamate release and mEPSCs amplitude [43–45]. In line with these observations, different acute stress protocols, as well as acute corticosterone in vivo and in vitro treatments, were shown to induce delayed and long-lasting increase of excitatory transmission in prefrontal cortex pyramidal neurons [44–46].
In recent studies, fast and slow effects of acute stress were also analyzed in the amygdala [47]. In basolateral amygdala, a brain area responsible for the control of emotion and fear memory, different and partially opposite effects of stress compared to hippocampus and prefrontal cortex were reported. Indeed, the early nongenomic increase of mEPSCs frequency induced by corticosterone is accompanied by a fast decrease in synaptic potentiation (LTP), while genomic and delayed effects include enhancement of neuronal excitability and synaptic plasticity.
Long-term adaptive changes induced by repeated stress exposure include impairments in neuronal transmission and synaptic activity (extensively reviewed in [27, 31, 32, 48]). Indeed, in both hippocampus and prefrontal cortex, chronic stress was associated with defects in the ability to induce or maintain LTP, and increased long-term depression (LTD), together with enhancing basal transmission, while opposite changes were measured in basolateral amygdala. The changes in neuronal activity induced by chronic stress in brain areas involved in the negative feedback of the HPA axis are consistent with impairment and dysregulation of the stress response [31]. It has been suggested that functional connectivity between amygdala and ventral hippocampus plays a key role in stress-induced changes in synaptic plasticity (for a recent review, see [49]). In particular, Ghosh and coworkers showed that, in animals subjected to repeated restraint stress, the directional connection from the amygdala to the hippocampus is gradually and persistently potentiated [50]. The authors suggest that this mechanism could be involved in the long-term emotional and cognitive impairments induced by chronic stress.
2.2. Morphological and Cytoarchitectural Changes Induced by Stress
Stress and corticosterone cause structural alterations, including dendritic remodeling and changes in spine density, mostly in brain areas implicated in the regulation of the emotional state (for recent reviews, see [27, 51, 52]).
Chronic stress was reported to reduce dendritic arborization and synaptic contacts both in hippocampus and in prefrontal cortex, whereas in basolateral amygdala, both chronic and acute exposure to stressors significantly increased dendritic complexity (for recent reviews, see [27, 48–53]). These long-lasting structural changes occur together with increased anxious- and depressive-like behaviors, strongly suggesting that dendritic atrophy induced by chronic stress may induce severe behavioral deficits [48, 53, 54].
Recently, a few studies also analyzed morphological alterations induced by acute stress and corticosterone. Acute corticosterone treatment of rats was shown to induce delayed and time-dependent opposite changes of dendrite morphology in medial prefrontal cortex pyramidal neurons, compared to basolateral amygdala spiny neurons [55]. Similarly, 5 hours of multimodal combined physical/psychological stress was demonstrated to induce a corticotropin-releasing hormone-dependent reduction of spine density in hippocampal area CA3 [54]. In a more recent study, one single session of acute footshock stress was shown to reduce the apical dendritic length of pyramidal neurons in medial prefrontal cortex layers II-III [56]. Intriguingly, this effect was measured as early as one day after stress and lasted for up to 14 days. Furthermore, the effect was partly prevented by chronic treatment with antidepressants before the stress session.
On the other hand, in line with the rapid enhancement of excitatory transmission induced by stress and improvement of working memory performance [44, 45], both acute footshock and acute restraint stress were shown to remarkably increase the number of excitatory axoshaft and axospinous synapses in the medial prefrontal cortex of rats [57] and to induce sprouting of new spines one day after stress [56]. Accordingly, Liston and Gan [58] have shown that acute treatment with corticosterone promotes a dose-dependent increase of spine formation in medial prefrontal cortex pyramidal neurons. Furthermore, acute corticosterone induces rapid GR-dependent spinogenesis in hippocampal slices [59]. These findings suggest that the increased number of synapses induced by acute stress is likely a corticosterone-dependent effect.
2.3. Epigenetic Changes Induced by Stress
The term “epigenetics” refers to mechanisms modulating gene expression independently of changes in nucleotide sequence and includes alterations of DNA methylation, posttranslational modification of histone proteins, and regulation by small noncoding RNAs (essentially, microRNAs, miR) [60, 61]. Compelling evidence showed that behavioral stress induces epigenetic changes in selected brain areas, leading to regulation of gene expression and neuronal function [61–63].
A few studies have assessed changes in DNA methylation (a modification associated with gene silencing) in stress animal models. Chronic social stress in mice induced persistent demethylation at the corticotrophin-releasing factor promoter in the paraventricular nucleus of the hypothalamus, suggesting hyperactivation of the HPA axis [64]. Moreover, in a recent study, changes in the global DNA methylation profile were measured after acute restraint stress in the hippocampus, cerebral cortex, and periaqueductal gray matter, while these alterations were prevented by physical exercise [65].
A growing body of literature reported posttranslational modification of histone proteins after exposure to acute and chronic stress protocols. A genome-wide chromatin immunoprecipitation study reported changes in histone H3 lysine 9 dimethylation levels (inducing repression of gene expression) in the nucleus accumbens of mice susceptible to chronic social defeat stress, and not in resilient animals [66, 67]; importantly, chronic antidepressants reversed these modifications [68]. A recent study also showed that both chronic social defeat in mice and depression in humans reduced the expression of the RAS-related C3 botulinum toxin substrate 1 (Rac1) gene in the nucleus accumbens, through a mechanism involving increased histone H3 lysine 9 dimethylation [69]. On the other hand, permissive histone H3 acetylation is transiently reduced and then persistently increased in the nucleus accumbens of susceptible, but not of resilient, animals subjected to chronic social stress [66]. In the same paper, similar results were obtained in postmortem studies, reporting increased levels of histone H3 acetylation in the nucleus accumbens of depressed patients. However, since local infusion of histone deacetylase inhibitors showed an antidepressant-like effect, the authors hypothesized that the increase in H3 acetylation measured in susceptible animals might mediate long-lasting positive neuronal adaptations to chronic stress.
In the hippocampus, selected and time-dependent changes in histone H3 methylation at lysines 4, 9, and 27 (resp., associated with increased transcription, heterochromatin formation, and transcriptional repression) were demonstrated after acute and repeated restraint stress in rats [70]. A further study from the same group showed that, soon after one single session of restraint stress, repressive histone H3 lysine 9 trimethylation is selectively increased in the hippocampus, especially at transposable element loci [71]. Individual variations of histone H3 acetylation levels were also reported in the hippocampus of rats subjected to repeated social defeat stress [72, 73]. Moreover, it was shown that the acquisition of behavioral immobility response induced by acute forced swim stress was dependent on increased histone H3 phosphoacetylation in the hippocampus and GR-induced activation of the NMDA/extracellular signal-regulated kinases (ERK)/mitogen- and stress-activated kinases (MSK) 1/2 pathway [74].
Repeated social defeat stress was also found to increase histone H3 acetylation in the infralimbic (and not prelimbic) prefrontal cortex [73, 75]. A recent study on postmortem PFC from patients with mood disorders reported increased levels of the presynaptic protein synapsin 2, together with increased histone H3 lysine 4 trimethylation at its promoter, suggesting epigenetic regulation of synapsin 2 gene expression [76].
Intriguingly, a number of studies reported stress-induced epigenetic regulation of the brain-derived neurotrophic factor (BDNF), a neurotrophin with key roles in neuroplasticity and synaptic function, as well as in the pathophysiology of neuropsychiatric disorders [77, 78]. The expression of BDNF is mediated by the transcription of different mRNAs, driven by dedicated promoters and derived by the splicing of one of multiple 5′ noncoding exons (at least eight in rodents) with the 3′ coding exon [79].
Social defeat stress induced long-lasting downregulation of BDNF transcripts containing exons IV and VI, by increasing dimethylation of histone H3 at specific exon promoters in the mouse hippocampus, and chronic imipramine reversed this downregulation increasing histone acetylation at the same promoters [80]. Similarly, the reduction of total BDNF transcript and mRNAs containing exons I and IV expression, induced by single immobilization stress in the rat hippocampus, was shown to be accompanied by a significant decrease in histone H3 acetylation at respective promoters [78]. In a more recent study, physical exercise was found to counteract the downregulation of selected BDNF transcripts induced by acute restraint stress and to increase the levels of histone H3 acetylation at related promoters [81].
MicroRNAs are small noncoding RNAs regulating gene expression, generally repressing the expression of target mRNAs [82]. In recent years, research studies have been conducted on the involvement of microRNAs in the stress response and onset of neuropsychiatric disorders [83]. It was shown that both acute restraint stress and chronic social defeat in mice markedly upregulated miR-34 levels in amygdala and that miR-34 overexpression in the central amygdala exerted anxiolytic effect [84]. In the same study, in vitro experiments showed that miR-34 reduced the activation of the corticotropin-releasing hormone receptor 1, suggesting a role of miR-34 in functional regulation of the stress response. In more recent papers from the same research group, miR-135 in serotonergic neurons was found to have a key role in determining stress resiliency and antidepressant efficacy [85], while the increase of amygdalar miR-19b induced by chronic social defeat stress was suggested to be related to behavioral responses to stress, through mechanisms involving the adrenergic receptor β-1 [86].
3. Perinatal Reprogramming of the Stress Response
The high majority of functional and morphological changes promoted by behavioral stress and corticosteroids were reported in “naïve” young adult animals or mature neuronal cultures. Nevertheless, early-life experiences shape the stress response in adulthood, leading to the reprogramming of coping strategies against environmental challenges and having a strong impact on behavior and susceptibility to neuropsychiatric disorders (see Section 1).
Intriguingly, a few studies on humans aimed at separating the effects of the objective exposure to a stressor and the mother's subjective reaction [87–90]. According to King and Laplante [87], exposure to a natural disaster (Project Ice Storm) occurring during the gestational period allows for a reliable study of the effects of prenatal stress on child health and development [91].
However, the reprogramming effects observed in the offspring likely recapitulate the cumulative experience in utero and the quality of the postnatal environment, which is, in turn, mostly associated with the quantity, quality, and reliability of maternal care [92–97]. Thus, we will refer to “perinatal” reprogramming to include events occurring prenatally and/or during the lactation period. Considering that the limitations of retrospective studies constrain the number of epidemiological findings in humans, a large number of data come from evidence in rodents and nonhuman primates [21, 24, 98–104].
3.1. Changes in Excitatory Neurotransmission Induced by Perinatal Stress
Overall, the changes in excitatory transmission and neuronal remodeling, induced by both acute and chronic stress (reviewed in Section 2.1), strongly suggest a key role of the glutamate synapse in the adaptive and maladaptive response to stressful stimuli. However, the study of the effects of exposure to perinatal stress on the activity of glutamatergic neurons is still at its infancy.
Morphological studies have shown that prenatal stress is associated with reduction of dendritic arborization and synaptic loss in prefrontal cortex and hippocampus in adult life, suggesting that stress in gestational period might induce long-lasting impairments of glutamate neuron and transmission [105–108].
A number of studies reported changes in the expression of glutamate receptors and transporters, in adult animals subjected to stress during the perinatal life [109–115]. Maternal separation in rats was found to decrease mRNA expression levels of ionotropic glutamate receptors, together with increasing GLutamate ASpartate Transporter (GLAST) levels, selectively in the hippocampus and not in the prefrontal cortex [109]. It was also demonstrated that maternal separation significantly reduced the expression of type 4 metabotropic glutamate receptor in hippocampus, a change reversed by chronic fluoxetine treatment [110]. Similarly, adult male offspring of pregnant dams subjected to restraint stress during pregnancy display impairment of N-methyl-D-aspartate (NMDA) receptor-mediated long-term potentiation, decreased NMDA receptor subunits [111], and reduced expression of group I/II metabotropic glutamate receptors [112] in the hippocampus. In a more recent study, Adrover and collaborators [113] have shown increased mRNA and protein expression levels of the glial glutamate transporter (GLT-1) in the hippocampus and enhanced glutamate uptake and vesicular glutamate transporter 1 (v-Glut-1) protein levels in the prefrontal cortex of prenatally stressed rats.
Overall, the high majority of studies reported that early stress both decreases the expression of glutamate receptors, suggesting reduced transmission efficacy, and increases glutamate transporters, which may imply an increased rate of glutamate metabolism. Of note, others have found higher levels of ionotropic and metabotropic glutamate receptors [114] and increased NMDA receptor activation [115]. Others have shown impairment of long-term potentiation and enhancement of long-term depression in young rats subjected to prenatal stress [116]. These abnormalities were correlated with increased pro-brain-derived neurotrophic factor (pro-BDNF), decreased mature BDNF levels, and no changes in NMDA receptor subunits expression [116]. Although changes in the expression of glutamate receptors and transporters are only rough indicators for predicting glutamate release and transmission, these data strongly suggest that perinatal stress exerts a long-term influence on the glutamate system.
It was recently demonstrated that the increase of anxiety-like behavior induced by prenatal stress in rats is causally associated with a reduction of depolarization-evoked presynaptic glutamate release in the ventral hippocampus [117, 118], a brain region encoding memories related to stress and emotions [119]. Interestingly, this effect is blocked by activation of oxytocin receptor [120] (see below for oxytocin and reprogramming). Although the mechanisms by which prenatal stress may cause long-lasting dampening of glutamate neurotransmission in the ventral hippocampus have been poorly clarified, it was hypothesized that prenatal stress, besides enhancing glutamate metabolism, might induce long-lasting dysfunction in the intrinsic machinery controlling exocytotic glutamate release [117].
3.2. Modulation of HPA Axis Reactivity Induced by Perinatal Stress
HPA axis alterations are the characteristic feature of the endophenotypes induced by perinatal stress [21, 121–126].
A pioneering study by Levine showed that maternal separation induced downregulation of the stress response, consistent with weight reduction of adrenal glands [127]. To date, the literature about the long-term effects of perinatal stress on the HPA axis is contradictory, although in many species including mice, rats, guinea pigs, and nonhuman primates, prenatal stress has been shown to increase the overall production of glucocorticoid and/or the feedback regulation [100, 101, 103, 128–130]. For example, peer rearing in monkeys has been shown to exaggerate stress reactivity [122, 123], stereotypies and self-directed behaviors [124], and abnormal brain morphology [125]. Moreover, maternal separated rodents show general upregulation of stress and fear responses [126, 131–133], increased hypothalamic CRF expression, reduced cortical GR expression [134], increased immobility in the forced-swim test [135], and poorer memory performance [134]. Curiously, in rats, 3 hours of daily maternal separation during the first two weeks after birth increases the vulnerability to stress in the adulthood [136, 137], whereas 8 hours of separation decreases the response of the HPA axis [138]. Similarly, prenatally restraint stressed rats display prolonged corticosterone secretion associated with downregulation of GR and MR receptors in the hippocampus [21, 92]. Interestingly, these effects are reversed by prenatal adrenalectomy [93] or postnatal cross-fostering [92].
3.3. Epigenetic Reprogramming of the HPA Axis: Regulation of GR Expression
An ever growing number of studies focused on short- and long-term epigenetic changes induced by stress in early life (recently extensively reviewed in [22, 92, 139–141]). The mechanisms involved in the epigenetic reprogramming are highly complex and strongly depend on the gender of the individual, the type of stressor, and its intensity and duration. Here, we will focus on the epigenetic regulation of GR in the offspring induced by prenatal and postnatal maternal stress.
At the epigenetic level, the GR gene is consistently affected by natural variation of maternal care in rodents (measured as licking/grooming, arched-back and blanket nursing, and nest building) [142, 143]. Indeed, low absolute levels of maternal care selectively modify the DNA methylation status of GR promoter in the hippocampus of the offspring, suggesting reduced expression of the receptor as well as increased HPA reactivity. Conversely, offspring receiving high levels of maternal care exhibit lower level of DNA methylation of the GR promoter and increased histone H3 lysine 9 acetylation (a marker of transcriptional activation).
The GR gene expression and promoter methylation have also been examined in humans following early-life trauma, with similar epigenetic outcomes. McGowan and collaborators [144] found decreased levels of hippocampal GR mRNA and increased cytosine methylation of the GR promoter in subjects with a history of childhood abuse. Similarly, childhood maltreatment has been associated with decreased hippocampal GR expression and increased stress responses in adulthood. Again, such effects are mediated by DNA methylation and hydroxymethylation across GR promoter regions [145]. A compelling study in genocide survivors suggested that the increased DNA methylation at the promoter region of the GR was associated with less intrusive memory of the traumatic event and sex-specific reduced PTSD risk [146]. Together, these findings indicate that the epigenetic regulation of GR expression is a key factor in the reprogramming of the HPA axis induced by early stress.
3.4. Mother-Offspring Interplay: Role of 11β-Hydroxysteroid Dehydrogenases and Oxytocin
The mother/infant interaction is a critical intermediary to study early-life reprogramming. Such interplay is mainly mediated by the placenta, which modulates fetal exposure to maternal factors. As an example, glucocorticoids, despite circulating across the placenta, are significantly lower in the fetus than in the mother. This key tissue-specific barrier control is exerted by the placental 11β-hydroxysteroid dehydrogenase, an enzyme that converts cortisol and corticosterone into inactive cortisone and 11-dehydrocorticosterone (11β-HSD2), and vice versa (11β-HSD1) [147–149]. Recently, it has been shown that 11β-HSD2 undergoes epigenetic regulation in the placenta and fetal brain [150–152]. Curiously, Appleton and collaborators [153] have shown that women experiencing adversity during pregnancy display low levels of 11β-HSD2 methylation. This accounts for increased levels of placental 11β-HSD aimed at protecting the fetus from excessive glucocorticoid exposure. Others have shown that pregnant rats exposed to repeated episodes of restraint stress, a model that recapitulates the main feature of anxiety and depression in the adult offspring, display a reduction of 11β-HSD2 activity in the placenta, thus increasing the amount of nonmetabolized corticosterone reaching the fetus [55]. Also, high methylation in the promoter region of placental 11β-HSD2 has been associated with low infant birth weight [154]. However, it is unclear whether the modifications of the 11β-HSD2 are exclusively disruptive and/or directly associated with pathological endophenotypes in late life. For example, the downregulation of 11β-HSD2 may provide the fetus with a reliable signal about the maternal stressful environment, thereby predicting the milieu it is likely to cope with after birth.
The maternal HPA axis itself also plays a pivotal role in the mother-offspring interplay. The HPA axis is normally attenuated from midpregnancy to the end of lactation [155–157]; such attenuation is generally associated with maternal behavioral changes including reduced anxiety [158, 159], enhanced maternal behavior [160], and increased aggressiveness [161, 162]. Of note, the central oxytocinergic system exerts this inhibitory effect on the maternal HPA axis [160, 163–167]. Oxytocin is a neurohypophysial peptide, which plays a key role in parturition, lactation, mother/infant interaction, and social behavior [168]. Interestingly, intracerebroventricular administration of oxytocin stimulates maternal behavior in ovariectomized virgin rats [160]. Moreover, enhanced maternal care increases the expression of oxytocin receptor in the central nucleus of the amygdala [169]. Remarkably, in subjects with a history of early-life stress, the inhibitory effect of oxytocin on the HPA axis is diminished or even reversed [120, 170]. Interestingly, impaired social behavior and increased anxiety have been associated with an altered number of oxytocin neurons in the paraventricular and supraoptic nuclei [171] and increased expression of oxytocin receptor in the hippocampus and amygdala [120] of adult prenatally stressed rats. Moreover, it has been shown that the activation of presynaptic oxytocin receptor during the adulthood could both correct the abnormal glucocorticoid feedback of the HPA axis and normalize the expression of GR and MR in the hippocampus in prenatally restraint stressed rats [120]. In humans, it has been shown that intranasal administration of oxytocin dampens the enhanced stress-induced functional connectivity between the amygdala and the hippocampus in subject with a history of early-life stress [172]. Finally, variations in maternal care have been associated with DNA methylation of oxytocin receptor in blood cells both in rodents [173] and in humans [174].
Together, the evidence reviewed in this section emphasizes the molecular and neuroendocrine mechanisms that underlie the critical role of mother-infant interaction in the reprogramming of stress response and vulnerability to neuropsychiatric disorders.
4. Conclusion
The “cumulative stress hypothesis” of neuropsychiatric disorders states that repeated exposure to stressful events is the main environmental factor for pathological onset [1, 8, 32]. Thus, it is conceivable that repeated adverse events, especially when added to perinatal stress, exacerbate psychopathological conditions. On the other hand, according to the “match/mismatch hypothesis,” early-life stress might also be somehow protective against stressors in late life, leading to higher achievement of adaptation and survival [7, 9, 175]. Yet, these hypotheses are strictly related and interconnected. Indeed, the deleterious effects of stress rely not only on when or how often stress occurs, but especially on how intense the stress is and how much it impacts an individual, depending on one's genetic background [176, 177]. In this context, repeated subjective mild stressors may act improving adaptation to environmental challenges [3, 178], while a single overwhelming adverse event may precipitate neuropsychiatric diseases, as in the case of posttraumatic stress disorder [179, 180].
It is also important to notice that whereas the physiological stress response activates adequate coping strategies, leading to stress resiliency and adaptation in the high majority of subjects, vulnerability toward stress is dependent on individual behavioral, physiological, and genetic factors [4–6]. Thus, individual reprogramming of the stress response induced by early-life stress could be both adaptive and maladaptive. In line with this hypothesis, Macrì and collaborators [181] have suggested that mild neonatal changes may reduce the HPA axis reactivity, leading to resilience, whereas severe neonatal challenges would increase the adult HPA axis reactivity, with the ensuing increased vulnerability to stress-related disorders. Intriguingly, it was recently reported that early-life trauma in humans can also promote early maturation of amygdala-prefrontal cortex connectivity, in line with enhanced emotion regulation and reduced anxious behavior [182].
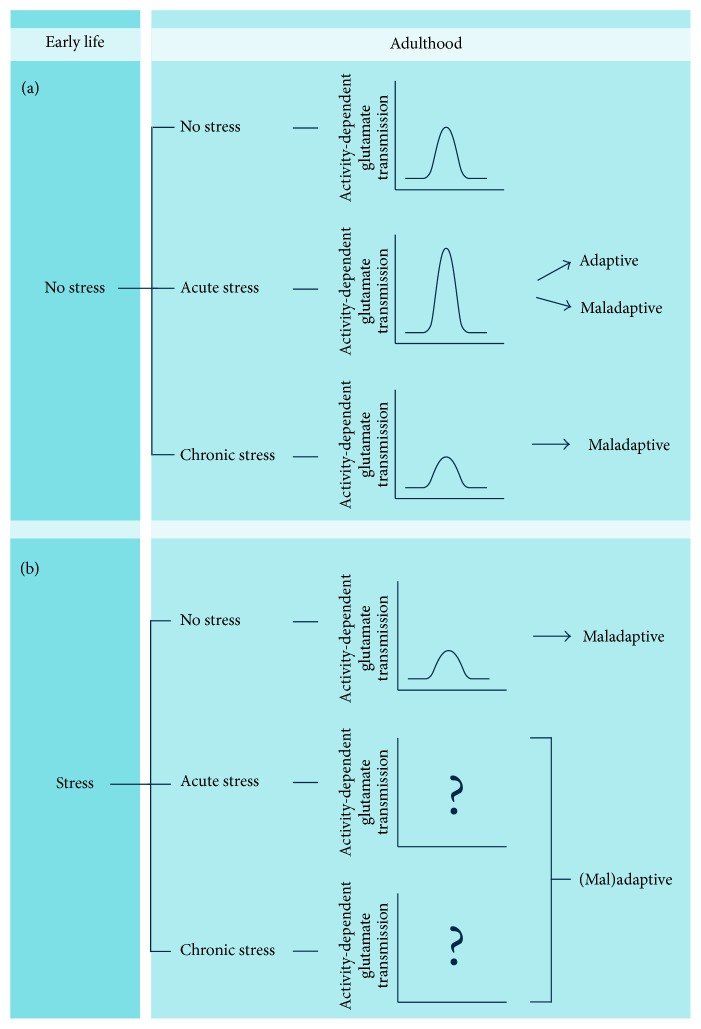
Integration of perinatal and late-life experiences may induce long-lasting consequences on neuronal excitatory transmission and morphology, especially in corticolimbic areas (Figure 1). If acute stress in adult life was consistently shown to increase glutamate transmission and release, at least in the hippocampus and prefrontal cortex [2, 26–28], chronic stress has the opposite effect, inducing impairments in neuronal transmission and synaptic activity [31] (Figure 1(a)). In turn, the effects of chronic stress are generally associated with behavioral deficits and depressive/anxious-like behavior [32, 61, 183], while the response to acute stress can be both adaptive, with improved behavioral and cognitive functions [44, 45], and maladaptive [7]. However, when the individual is subjected to stress in early life, the stress response in adulthood may be shaped by prior experiences (Figure 1(b)). It was shown that depolarization-dependent release of glutamate in dorsal hippocampus is decreased in animals subjected to chronic stress in the prenatal life [117, 118]; however, little is known on the reprogramming of the stress response at the level of excitatory transmission induced by early-life stress. A very recent cross-sectional observational study examined the effects of early- and late-life trauma in Korean college students, showing a significant correlation between early trauma, stress, and psychological distress [184].
Figure 1.
Influence of early-life stress on neuronal excitatory neurotransmission in corticolimbic areas. (a) In subjects with no history of perinatal adverse challenges, acute stress induces an increase in stimulation-evoked glutamate release. This response can be both adaptive and maladaptive. On the other hand, exposure to repeated episodes of stress (chronic stress) induces hypofunction of the glutamatergic synapse with reduced evoked glutamate release, associated with increased vulnerability to stress-related neuropsychiatric disorders. (b) Perinatal stress induces hypofunction of the glutamatergic synapse in adult life, with ensuing reduction in evoked glutamate release. The effects of the association between early- and late-life stress are largely unknown. See text for details.
We speculate that the long-lasting attenuation of the stress response induced by early-life stress might also affect the changes in excitatory transmission usually induced by stress in adult life. Thus, hypothetically, both the rise of glutamate transmission induced by acute stress and the attenuation of excitatory currents caused by chronic stress might be affected by reprogramming of the stress response induced by early-life stress, thus likely leading to adaptive or maladaptive changes, depending on the intensities of the stressors. Experimental evidence is required to validate or falsify the hypothesis.
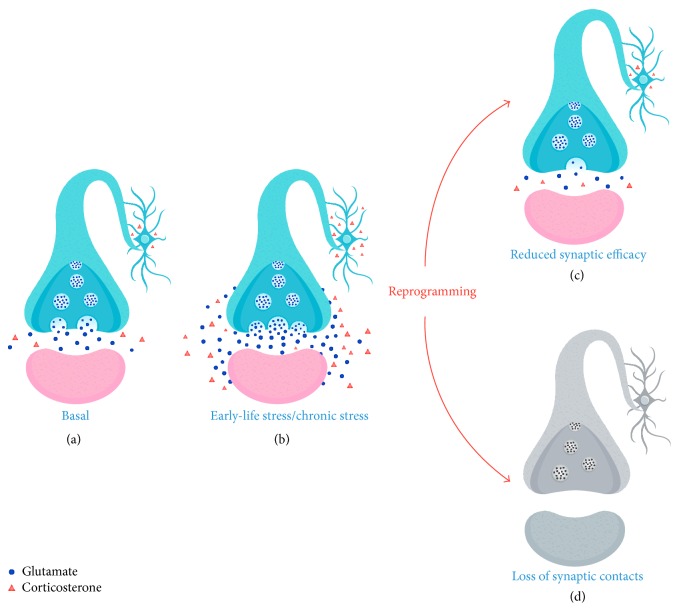
Excitotoxicity caused by excessive glutamate release and epigenetic reprogramming are reasonably among the main mechanisms involved in long-lasting neuroplastic alterations induced by stress [1, 185] (Figure 2). Excitoxicity is generally associated with reduced ability to clear the synaptic glutamate, resulting in glutamate spillover and activation of extrasynaptic glutamate receptors [32]. However, it is likely that exposure to high levels of corticosteroids (Figure 2(b)), together with inducing changes in excitatory transmission, activates epigenetic mechanisms, which modulate gene expression and neuronal responsiveness to stress. Of note, mounting evidence suggests that perinatal stress reprogramming of the neuroendocrine stress response and the ensuing behavioral state can cross multiple generations, thus supporting the hypothesis that epigenetic mechanisms underlie the reprogramming of the “stressed synapse” [22, 186]. This in turn leads to functional and structural consequences, in line with reduced synaptic efficacy [31, 117, 118] (Figure 2(c)) and number of synaptic contacts [52, 53, 105–108] (Figure 2(d)).
Figure 2.
Long-term neuroplastic alterations induced by early-life stress and chronic stress. (a) Basal condition: presynaptic neuron (light blue), postsynaptic neuron (pink). (b) Repeated episodes of stress in early life or in adulthood induce an increase in glucocorticoids associated with a transient increase in glutamate release both in the synaptic cleft and in the extrasynaptic space. Increase in glutamate release may activate reprogramming mechanisms that lead to either reduced synaptic efficacy (c) or loss of synaptic contacts (d).
The mechanisms by which early-life events affect stress resilience via the reprogramming of the stress response and the modulation of excitatory neurotransmission warrant further investigation. In-depth studies of changes in glutamate transmission and dendrite remodeling induced by stress in early and late life will help to elucidate the biological underpinnings of the (mal)adaptive strategies the brain adopts to cope with environmental challenges in one's life.
Acknowledgments
The authors would like to thank Fabio Persico for illustrations and graphical layout and Elisabeth Donnelly for editing and proofreading. Special thanks are due to Professors Bruce S. McEwen and Maurizio Popoli for their valued comments and suggestions.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.McEwen B. S. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124. doi: 10.1016/s0893-133x(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 2.Myers B., McKlveen J. M., Herman J. P. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Frontiers in Neuroendocrinology. 2014;35(2):180–196. doi: 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchoa E. T., Aguilera G., Herman J. P., Fiedler J. L., Deak T., de Sousa M. B. C. Novel aspects of glucocorticoid actions. Journal of Neuroendocrinology. 2014;26(9):557–572. doi: 10.1111/jne.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obradović J., Boyce W. T. Individual differences in behavioral, physiological, and genetic sensitivities to contexts: implications for development and adaptation. Developmental Neuroscience. 2009;31(4):300–308. doi: 10.1159/000216541. [DOI] [PubMed] [Google Scholar]
- 5.Russo S. J., Murrough J. W., Han M.-H., Charney D. S., Nestler E. J. Neurobiology of resilience. Nature Neuroscience. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin T. B., Saab B. J., Mansuy I. M. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75(5):747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Daskalakis N. P., Bagot R. C., Parker K. J., Vinkers C. H., de Kloet E. R. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38(9):1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen B. S. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583(2-3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M. V. Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology. 2011;36(3):330–338. doi: 10.1016/j.psyneuen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Santarelli S., Lesuis S. L., Wang X.-D., et al. Evidence supporting the match/mismatch hypothesis of psychiatric disorders. European Neuropsychopharmacology. 2014;24(6):907–918. doi: 10.1016/j.euroneuro.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Stott D. H. Follow up study from birth of the effects of prenatal stresses. Developmental Medicine and Child Neurology. 1973;15(6):770–787. doi: 10.1111/j.1469-8749.1973.tb04912.x. [DOI] [PubMed] [Google Scholar]
- 12.Blomberg S. Influence of maternal distress during pregnancy on postnatal development. Acta Psychiatrica Scandinavica. 1980;62(5):405–417. doi: 10.1111/j.1600-0447.1980.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 13.Ward I. L., Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114(5):1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- 14.Meijer A. Child psychiatric sequelae of maternal war stress. Acta Psychiatrica Scandinavica. 1985;72(6):505–511. doi: 10.1111/j.1600-0447.1985.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 15.Homer C. J., Beresford S. A. A., James S. A., Siegel E., Wilcox S. Work-related physical exertion and risk of preterm, low birthweight delivery. Paediatric and Perinatal Epidemiology. 1990;4(2):161–174. doi: 10.1111/j.1365-3016.1990.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 16.Holmes L. B. Looking for long-term effects from prenatal exposures to anticonvulsants. Teratology. 2001;64(4):175–176. doi: 10.1002/tera.1061. [DOI] [PubMed] [Google Scholar]
- 17.Weinstock M. Effects of maternal stress on development and behaviour in rat offspring. Stress. 2001;4(3):157–167. doi: 10.3109/10253890109035015. [DOI] [PubMed] [Google Scholar]
- 18.Darnaudéry M., Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Research Reviews. 2008;57(2):571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Lupien S. J., McEwen B. S., Gunnar M. R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 20.Brunton P. J., Russell J. A. Endocrine induced changes in brain function during pregnancy. Brain Research. 2010;1364:198–215. doi: 10.1016/j.brainres.2010.09.062. [DOI] [PubMed] [Google Scholar]
- 21.Maccari S., Krugers H. J., Morley-Fletcher S., Szyf M., Brunton P. J. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. Journal of Neuroendocrinology. 2014;26(10):707–723. doi: 10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- 22.Bale T. L. Epigenetic and transgenerational reprogramming of brain development. Nature Reviews Neuroscience. 2015;16(6):332–344. doi: 10.1038/nrn3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gee D. G., Casey B. J. The impact of developmental timing for stress and recovery. Neurobiology of Stress. 2015;1:184–194. doi: 10.1016/j.ynstr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meaney M. J. Effects of the social environment and early life stress on neurodevelopment, cognition, behaviour and health. Psychoneuroendocrinology. 2015;61:p. 11. doi: 10.1016/j.psyneuen.2015.07.418. [DOI] [Google Scholar]
- 25.Groeneweg F. L., Karst H., de Kloet E. R., Joëls M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Molecular and Cellular Endocrinology. 2012;350(2):299–309. doi: 10.1016/j.mce.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Joëls M., Pasricha N., Karst H. The interplay between rapid and slow corticosteroid actions in brain. European Journal of Pharmacology. 2013;719(1–3):44–52. doi: 10.1016/j.ejphar.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Musazzi L., Treccani G., Popoli M. Functional and structural remodeling of glutamate synapses in prefrontal and frontal cortex induced by behavioral stress. Frontiers in Psychiatry. 2015;6, article 60 doi: 10.3389/fpsyt.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timmermans W., Xiong H., Hoogenraad C. C., Krugers H. J. Stress and excitatory synapses: from health to disease. Neuroscience. 2013;248:626–636. doi: 10.1016/j.neuroscience.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 29.Groc L., Choquet D., Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nature Neuroscience. 2008;11(8):868–870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- 30.Kim J. J., Diamond D. M. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 31.Joëls M., Sarabdjitsingh R. A., Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacological Reviews. 2012;64(4):901–938. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- 32.Popoli M., Yan Z., McEwen B. S., Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature Reviews Neuroscience. 2012;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Research Reviews. 2009;61(2):105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Maguire J. Stress-induced plasticity of GABAergic inhibition. Frontiers in Cellular Neuroscience. 2014;8, article 157 doi: 10.3389/fncel.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karst H., Berger S., Turiault M., Tronche F., Schütz G., Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(52):19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olijslagers J. E., De Kloet E. R., Elgersma Y., Van Woerden G. M., Joëls M., Karst H. Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. European Journal of Neuroscience. 2008;27(10):2542–2550. doi: 10.1111/j.1460-9568.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- 37.Pasricha N., Joëls M., Karst H. Rapid effects of corticosterone in the mouse dentate gyrus via a nongenomic pathway. Journal of Neuroendocrinology. 2011;23(2):143–147. doi: 10.1111/j.1365-2826.2010.02091.x. [DOI] [PubMed] [Google Scholar]
- 38.Karst H., Joëls M. Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. Journal of Neurophysiology. 2005;94(5):3479–3486. doi: 10.1152/jn.00143.2005. [DOI] [PubMed] [Google Scholar]
- 39.Martin S., Henley J. M., Holman D., et al. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS ONE. 2009;4(3) doi: 10.1371/journal.pone.0004714.e4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C.-C., Yang C.-H., Huang C.-C., Hsu K.-S. Acute stress impairs hippocampal mossy fiber-CA3 long-term potentiation by enhancing cAMP-specific phosphodiesterase 4 activity. Neuropsychopharmacology. 2010;35(7):1605–1617. doi: 10.1038/npp.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musazzi L., Milanese M., Farisello P., et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS ONE. 2010;5(1) doi: 10.1371/journal.pone.0008566.e8566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treccani G., Musazzi L., Perego C., et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Molecular Psychiatry. 2014;19(4):433–443. doi: 10.1038/mp.2014.5. [DOI] [PubMed] [Google Scholar]
- 43.Treccani G., Musazzi L., Perego C., et al. Acute stress rapidly increases the readily releasable pool of glutamate vesicles in prefrontal and frontal cortex through non-genomic action of corticosterone. Molecular Psychiatry. 2014;19(4, article 401) doi: 10.1038/mp.2014.20. [DOI] [PubMed] [Google Scholar]
- 44.Yuen E. Y., Liu W., Karatsoreos I. N., Feng J., McEwen B. S., Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(33):14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuen E. Y., Liu W., Karatsoreos I. N., et al. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Molecular Psychiatry. 2011;16(2):156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J. B., Wei J., Liu W., Cheng J., Feng J., Yan Z. Histone deacetylase 6 gates the synaptic action of acute stress in prefrontal cortex. Journal of Physiology. 2012;590(7):1535–1546. doi: 10.1113/jphysiol.2011.224907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarabdjitsingh R. A., Joëls M. Rapid corticosteroid actions on synaptic plasticity in the mouse basolateral amygdala: relevance of recent stress history and β-adrenergic signaling. Neurobiology of Learning and Memory. 2014;112:168–175. doi: 10.1016/j.nlm.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Radley J., Morilak D., Viau V., Campeau S. Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neuroscience & Biobehavioral Reviews. 2015;58:79–91. doi: 10.1016/j.neubiorev.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duman C. H., Duman R. S. Spine synapse remodeling in the pathophysiology and treatment of depression. Neuroscience Letters. 2015;601:20–29. doi: 10.1016/j.neulet.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh S., Laxmi T. R., Chattarji S. Functional connectivity from the amygdala to the hippocampus grows stronger after stress. Journal of Neuroscience. 2013;33(17):7234–7244. doi: 10.1523/jneurosci.0638-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leuner B., Shors T. J. Stress, anxiety, and dendritic spines: what are the connections? Neuroscience. 2013;251:108–119. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 52.Arnsten A. F. Stress weakens prefrontal networks: molecular insults to higher cognition. Nature Neuroscience. 2015;18(10):1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Licznerski P., Duman R. S. Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience. 2013;251:33–50. doi: 10.1016/j.neuroscience.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y., Rex C. S., Rice C. J., et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim H., Yi J. H., Choi K., Hong S., Shin K. S., Kang S. J. Regional differences in acute corticosterone-induced dendritic remodeling in the rat brain and their behavioral consequences. BMC Neuroscience. 2014;15, article 65 doi: 10.1186/1471-2202-15-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nava N., Treccani G., Alabsi A., et al. Temporal dynamics of acute stress-induced dendritic remodeling in medial prefrontal cortex and the protective effect of desipramine. Cerebral Cortex. 2015 doi: 10.1093/cercor/bhv254. [DOI] [PubMed] [Google Scholar]
- 57.Nava N., Treccani G., Liebenberg N., et al. Chronic desipramine prevents acute stress-induced reorganization of medial prefrontal cortex architecture by blocking glutamate vesicle accumulation and excitatory synapse increase. International Journal of Neuropsychopharmacology. 2015;18(3) doi: 10.1093/ijnp/pyu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liston C., Gan W.-B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(38):16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komatsuzaki Y., Hatanaka Y., Murakami G., et al. Corticosterone induces rapid spinogenesis via synaptic glucocorticoid receptors and kinase networks in hippocampus. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0034124.e34124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tardito D., Mallei A., Popoli M. Lost in translation. New unexplored avenues for neuropsychopharmacology: epigenetics and microRNAs. Expert Opinion on Investigational Drugs. 2013;22(2):217–233. doi: 10.1517/13543784.2013.749237. [DOI] [PubMed] [Google Scholar]
- 61.Bagot R. C., Labonté B., Peña C. J., Nestler E. J. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues in Clinical Neuroscience. 2014;16(3):281–295. doi: 10.31887/DCNS.2014.16.3/rbagot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuchikami M., Yamamoto S., Morinobu S., Takei S., Yamawaki S. Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investigation. 2010;7(4):251–256. doi: 10.4306/pi.2010.7.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McEwen B. S., Nasca C., Gray J. D. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2015;41(1):3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elliott E., Ezra-Nevo G., Regev L., Neufeld-Cohen A., Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nature Neuroscience. 2010;13(11):1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- 65.Rodrigues G. M., Jr., Toffoli L. V., Manfredo M. H., et al. Acute stress affects the global DNA methylation profile in rat brain: modulation by physical exercise. Behavioural Brain Research. 2015;279:123–128. doi: 10.1016/j.bbr.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 66.Covington H. E., III, Maze I., LaPlant Q. C., et al. Antidepressant actions of histone deacetylase inhibitors. Journal of Neuroscience. 2009;29(37):11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Covington H. E., Vialou V. F., LaPlant Q., Ohnishi Y. N., Nestler E. J. Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neuroscience Letters. 2011;493(3):122–126. doi: 10.1016/j.neulet.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilkinson M. B., Xiao G., Kumar A., et al. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. Journal of Neuroscience. 2009;29(24):7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Golden S. A., Christoffel D. J., Heshmati M., et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nature Medicine. 2013;19(3):337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hunter R. G., McCarthy K. J., Milne T. A., Pfaff D. W., McEwen B. S. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(49):20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunter R. G., Murakami G., Dewell S., et al. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(43):17657–17662. doi: 10.1073/pnas.1215810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hollis F., Duclot F., Gunjan A., Kabbaj M. Individual differences in the effect of social defeat on anhedonia and histone acetylation in the rat hippocampus. Hormones and Behavior. 2011;59(3):331–337. doi: 10.1016/j.yhbeh.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kenworthy C. A., Sengupta A., Luz S. M., et al. Social defeat induces changes in histone acetylation and expression of histone modifying enzymes in the ventral hippocampus, prefrontal cortex, and dorsal raphe nucleus. Neuroscience. 2014;264:88–98. doi: 10.1016/j.neuroscience.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 74.Chandramohan Y., Droste S. K., Arthur J. S. C., Reul J. M. H. M. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. European Journal of Neuroscience. 2008;27(10):2701–2713. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- 75.Hinwood M., Tynan R. J., Day T. A., Walker F. R. Repeated social defeat selectively increases δfosB expression and histone h3 acetylation in the infralimbic medial prefrontal cortex. Cerebral Cortex. 2011;21(2):262–271. doi: 10.1093/cercor/bhq080. [DOI] [PubMed] [Google Scholar]
- 76.Cruceanu C., Alda M., Nagy C., Freemantle E., Rouleau G. A., Turecki G. H3K4 tri-methylation in synapsin genes leads to different expression patterns in bipolar disorder and major depression. International Journal of Neuropsychopharmacology. 2013;16(2):289–299. doi: 10.1017/S1461145712000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vialou V., Feng J., Robison A. J., Nestler E. J. Epigenetic mechanisms of depression and antidepressant action. Annual Review of Pharmacology and Toxicology. 2013;53:59–87. doi: 10.1146/annurev-pharmtox-010611-134540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fuchikami M., Morinobu S., Kurata A., Yamamoto S., Yamawaki S. Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. International Journal of Neuropsychopharmacology. 2009;12(1):73–82. doi: 10.1017/S1461145708008997. [DOI] [PubMed] [Google Scholar]
- 79.Aid T., Kazantseva A., Piirsoo M., Palm K., Timmusk T. Mouse and rat BDNF gene structure and expression revisited. Journal of Neuroscience Research. 2007;85(3):525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsankova N. M., Berton O., Renthal W., Kumar A., Neve R. L., Nestler E. J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature Neuroscience. 2006;9(4):519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 81.Ieraci A., Mallei A., Musazzi L., Popoli M. Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice. Hippocampus. 2015;25(11):1380–1392. doi: 10.1002/hipo.22458. [DOI] [PubMed] [Google Scholar]
- 82.Pasquinelli A. E. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nature Reviews Genetics. 2012;13(4):271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 83.Issler O., Chen A. Determining the role of microRNAs in psychiatric disorders. Nature Reviews Neuroscience. 2015;16(4):201–212. doi: 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- 84.Haramati S., Navon I., Issler O., et al. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. Journal of Neuroscience. 2011;31(40):14191–14203. doi: 10.1523/jneurosci.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Issler O., Haramati S., Paul E. D., et al. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron. 2014;83(2):344–360. doi: 10.1016/j.neuron.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 86.Volk N., Paul E. D., Haramati S., et al. MicroRNA-19b associates with Ago2 in the amygdala following chronic stress and regulates the adrenergic receptor beta 1. The Journal of Neuroscience. 2014;34(45):15070–15082. doi: 10.1523/jneurosci.0855-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.King S., Laplante D. P. The effects of prenatal maternal stress on children's cognitive development: project ice storm. Stress. 2005;8(1):35–45. doi: 10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- 88.Cao-Lei L., Massart R., Suderman M. J., et al. DNA methylation signatures triggered by prenatal maternal stress exposure to a natural disaster: Project ice storm. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0107653.e107653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cao-Lei L., Elgbeili G., Massart R., Laplante D. P., Szyf M., King S. Pregnant women's cognitive appraisal of a natural disaster affects DNA methylation in their children 13 years later: Project Ice Storm. Translational Psychiatry. 2015;2, article e515 doi: 10.1038/tp.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laplante D. P., Brunet A., King S. The effects of maternal stress and illness during pregnancy on infant temperament: project Ice Storm. Pediatric Research. 2015;79:107–113. doi: 10.1038/pr.2015.177. [DOI] [PubMed] [Google Scholar]
- 91.King S., Dancause K., Turcotte-Tremblay A.-M., Veru F., Laplante D. P. Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Research Part C—Embryo Today: Reviews. 2012;96(4):273–288. doi: 10.1002/bdrc.21026. [DOI] [PubMed] [Google Scholar]
- 92.Maccari S., Piazza P. V., Kabbaj M., Barbazanges A., Simon H., Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. The Journal of Neuroscience. 1995;15(1, part 1):110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barbazanges A., Piazza P. V., Le Moal M., Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. The Journal of Neuroscience. 1996;16(12):3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brown A. S., Susser E. S. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophrenia Bulletin. 2008;34(6):1054–1063. doi: 10.1093/schbul/sbn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown A. S., Vinogradov S., Kremen W. S., et al. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. The American Journal of Psychiatry. 2009;166(6):683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown A. S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Developmental Neurobiology. 2012;72(10):1272–1276. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meaney M. J. Epigenetics and the biological definition of gene X environment interactions. Child Development. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 98.Lyons D. M., Martel F. L., Levine S., Risch N. J., Schatzberg A. F. Postnatal experiences and genetic effects on squirrel monkey social affinities and emotional distress. Hormones and Behavior. 1999;36(3):266–275. doi: 10.1006/hbeh.1999.1547. [DOI] [PubMed] [Google Scholar]
- 99.Sánchez M. M., Ladd C. O., Plotsky P. M. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 100.Schneider M. L., Moore C. F., Kraemer G. W., Roberts A. D., DeJesus O. T. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27(1-2):285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- 101.Coe C. L., Kramer M., Czéh B., et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile Rhesus monkeys. Biological Psychiatry. 2003;54(10):1025–1034. doi: 10.1016/S0006-3223(03)00698-X. [DOI] [PubMed] [Google Scholar]
- 102.Mueller B. R., Bale T. L. Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of Neuroscience. 2008;28(36):9055–9065. doi: 10.1523/jneurosci.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kapoor A., Kostaki A., Janus C., Matthews S. G. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behavioural Brain Research. 2009;197(1):144–149. doi: 10.1016/j.bbr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 104.Korosi A., Baram T. Z. Plasticity of the stress response early in life: mechanisms and significance. Developmental Psychobiology. 2010;52(7):661–670. doi: 10.1002/dev.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martínez-Téllez R. I., Hernández-Torres E., Gamboa C., Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63(9):794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- 106.Barros V. G., Duhalde-Vega M., Caltana L., Brusco A., Antonelli M. C. Astrocyte-neuron vulnerability to prenatal stress in the adult rat brain. Journal of Neuroscience Research. 2006;83(5):787–800. doi: 10.1002/jnr.20758. [DOI] [PubMed] [Google Scholar]
- 107.Murmu M. S., Salomon S., Biala Y., Weinstock M., Braun K., Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. European Journal of Neuroscience. 2006;24(5):1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- 108.Suenaga T., Yukie M., Gao S., Nakahara D. Sex-specific effects of prenatal stress on neuronal development in the medial prefrontal cortex and the hippocampus. NeuroReport. 2012;23(7):430–435. doi: 10.1097/WNR.0b013e3283529805. [DOI] [PubMed] [Google Scholar]
- 109.Pickering C., Gustafsson L., Cebere A., Nylander I., Liljequist S. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Research. 2006;1099(1):101–108. doi: 10.1016/j.brainres.2006.04.136. [DOI] [PubMed] [Google Scholar]
- 110.O'Connor R. M., Pusceddu M. M., Dinan T. G., Cryan J. F. Impact of early-life stress, on group III mGlu receptor levels in the rat hippocampus: effects of ketamine, electroconvulsive shock therapy and fluoxetine treatment. Neuropharmacology. 2013;66:236–241. doi: 10.1016/j.neuropharm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 111.Son G. H., Geum D., Chung S., et al. Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity. The Journal of Neuroscience. 2006;26(12):3309–3318. doi: 10.1523/jneurosci.3850-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zuena A. R., Mairesse J., Casolini P., et al. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS ONE. 2008;3(5) doi: 10.1371/journal.pone.0002170.e2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Adrover E., Pallarés M. E., Baier C. J., et al. Glutamate neurotransmission is affected in prenatally stressed offspring. Neurochemistry International. 2015;88:73–87. doi: 10.1016/j.neuint.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 114.Berger M. A., Barros V. G., Sarchi M. I., Tarazi F. I., Antonelli M. C. Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochemical Research. 2002;27(11):1525–1533. doi: 10.1023/A:1021656607278. [DOI] [PubMed] [Google Scholar]
- 115.Barros V. G., Berger M. A., Martijena I. D., et al. Early adoption modifies the effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Journal of Neuroscience Research. 2004;76(4):488–496. doi: 10.1002/jnr.20119. [DOI] [PubMed] [Google Scholar]
- 116.Yeh C.-M., Huang C.-C., Hsu K.-S. Prenatal stress alters hippocampal synaptic plasticity in young rat offspring through preventing the proteolytic conversion of pro-brain-derived neurotrophic factor (BDNF) to mature BDNF. The Journal of Physiology. 2012;590(4):991–1010. doi: 10.1113/jphysiol.2011.222042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marrocco J., Mairesse J., Ngomba R. T., et al. Anxiety-like behavior of prenatally stressed rats is associated with a selective reduction of glutamate release in the ventral hippocampus. The Journal of Neuroscience. 2012;32(48):17143–17154. doi: 10.1523/jneurosci.1040-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marrocco J., Reynaert M.-L., Gatta E., et al. The effects of antidepressant treatment in prenatally stressed rats support the glutamatergic hypothesis of stress-related disorders. The Journal of Neuroscience. 2014;34(6):2015–2024. doi: 10.1523/jneurosci.4131-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fanselow M. S., Dong H.-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mairesse J., Gatta E., Reynaert M., et al. Activation of presynaptic oxytocin receptors enhances glutamate release in the ventral hippocampus of prenatally restraint stressed rats. Psychoneuroendocrinology. 2015;62:36–46. doi: 10.1016/j.psyneuen.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 121.Welberg L. A. M., Seckl J. R., Holmes M. C. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104(1):71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 122.Suomi S. J. Early stress and adult emotional reactivity in rhesus monkeys. Ciba Foundation Symposium. 1991;156:171–188. doi: 10.1002/9780470514047.ch11. [DOI] [PubMed] [Google Scholar]
- 123.Contia G., Hansman C., Heckman J. J., Novak M. F. X., Ruggiero A., Suomi S. J. Primate evidence on the late health effects of early-life adversity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(23):8866–8871. doi: 10.1073/pnas.1205340109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cirulli F., Francia N., Branchi I., et al. Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology. 2009;34(2):172–180. doi: 10.1016/j.psyneuen.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spinelli S., Chefer S., Suomi S. J., Higley J. D., Barr C. S., Stein E. Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry. 2009;66(6):658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huot R. L., Gonzalez M. E., Ladd C. O., Thrivikraman K. V., Plotsky P. M. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004;29(2):279–289. doi: 10.1016/S0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 127.Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126(3270, article 405) doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- 128.Lemaire V., Koehl M., Le Moal M., Abrous D. N. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Welberg L. A. M., Seckl J. R. Prenatal stress, glucocorticoids and the programming of the brain. Journal of Neuroendocrinology. 2001;13(2):113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 130.Brunton P. J. Programming the brain and behaviour by early-life stress: a focus on neuroactive steroids. Journal of Neuroendocrinology. 2015;27(6):468–480. doi: 10.1111/jne.12265. [DOI] [PubMed] [Google Scholar]
- 131.Macrì S., Laviola G. Single episode of maternal deprivation and adult depressive profile in mice: interaction with cannabinoid exposure during adolescence. Behavioural Brain Research. 2004;154(1):231–238. doi: 10.1016/j.bbr.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 132.Plotsky P. M., Meaney M. J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18(3):195–200. doi: 10.1016/0169-328X(93)90189-V. [DOI] [PubMed] [Google Scholar]
- 133.McCormick C. M., Kehoe P., Kovacs S. Corticosterone release in response to repeated, short episodes of neonatal isolation: evidence of sensitization. International Journal of Developmental Neuroscience. 1998;16(3-4):175–185. doi: 10.1016/s0736-5748(98)00026-4. [DOI] [PubMed] [Google Scholar]
- 134.Huot R. L., Plotsky P. M., Lenox R. H., McNamara R. K. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Research. 2002;950(1-2):52–63. doi: 10.1016/S0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- 135.Lee J.-H., Kim H. J., Kim J. G., et al. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neuroscience Research. 2007;58(1):32–39. doi: 10.1016/j.neures.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 136.Daniels W. M. U., Pietersen C. Y., Carstens M. E., Stein D. J. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metabolic Brain Disease. 2004;19(1-2):3–14. doi: 10.1023/B:MEBR.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- 137.Uchida S., Hara K., Kobayashi A., et al. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. The Journal of Neuroscience. 2010;30(45):15007–15018. doi: 10.1523/jneurosci.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Enthoven L., Oitzl M. S., Koning N., van der Mark M., de Kloet E. R. Hypothalamic-pituitary-adrenal axis activity of newborn mice rapidly desensitizes to repeated maternal absence but becomes highly responsive to novelty. Endocrinology. 2008;149(12):6366–6377. doi: 10.1210/en.2008-0238. [DOI] [PubMed] [Google Scholar]
- 139.Kundakovic M., Champagne F. A. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology. 2015;40(1):141–153. doi: 10.1038/npp.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Provençal N., Binder E. B. The effects of early life stress on the epigenome: from the womb to adulthood and even before. Experimental Neurology. 2015;268:10–20. doi: 10.1016/j.expneurol.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 141.Babenko O., Kovalchuk I., Metz G. A. S. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neuroscience and Biobehavioral Reviews. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 142.Weaver I. C. G., Cervoni N., Champagne F. A., et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 143.Weaver I. C. G. Epigenetic programming by maternal behavior and pharmacological intervention—nature versus nurture: let's call the whole thing off. Epigenetics. 2007;2(1):22–28. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- 144.McGowan P. O., Sasaki A., D'Alessio A. C., et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang T. Y., Labonté B., Wen X. L., Turecki G., Meaney M. J. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. 2013;38(1):111–123. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vukojevic V., Kolassa I.-T., Fastenrath M., et al. Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors. The Journal of Neuroscience. 2014;34(31):10274–10284. doi: 10.1523/jneurosci.1526-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Amelung D., Hubener H. J., Roka L., Meyerheim G. Conversion of cortisone to compound F. The Journal of Clinical Endocrinology and Metabolism. 1953;13(9):1125–1126. doi: 10.1210/jcem-13-9-1125. [DOI] [PubMed] [Google Scholar]
- 148.Seckl J. R., Chapman K. E. The 11β-hydroxysteroid dehydrogenase system, a determinant of glucocorticoid and mineralocorticoid action: medical and physiological aspects of the 11β-hydroxysteroid dehydrogenase system. European Journal of Biochemistry. 1997;249(2):361–364. doi: 10.1111/j.1432-1033.1997.t01-1-00361.x. [DOI] [PubMed] [Google Scholar]
- 149.Seckl J. R., Walker B. R. Minireview: 11β-hydroxysteroid dehydrogenase type 1—a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142(4):1371–1376. doi: 10.1210/en.142.4.1371. [DOI] [PubMed] [Google Scholar]
- 150.Jensen Peña C., Monk C., Champagne F. A. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0039791.e39791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Togher K. L., O'Keeffe M. M., Khashan A. S., Gutierrez H., Kenny L. C., O'Keeffe G. W. Epigenetic regulation of the placental HSD11B2 barrier and its role as a critical regulator of fetal development. Epigenetics. 2014;9(6):816–822. doi: 10.4161/epi.28703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nugent B. M., Bale T. L. The omniscient placenta: metabolic and epigenetic regulation of fetal programming. Frontiers in Neuroendocrinology. 2015;39:28–37. doi: 10.1016/j.yfrne.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Appleton A. A., Armstrong D. A., Lesseur C., et al. Patterning in placental 11-B hydroxysteroid dehydrogenase methylation according to prenatal socioeconomic adversity. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0074691.e74691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marsit C. J., Maccani M. A., Padbury J. F., Lester B. M. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0033794.e33794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stern J. M., Goldman L., Levine S. Pituitary adrenal responsiveness during lactation in rats. Neuroendocrinology. 1973;12(3):179–191. doi: 10.1159/000122167. [DOI] [PubMed] [Google Scholar]
- 156.Neumann I. D., Johnstone H. A., Hatzinger M., et al. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. The Journal of Physiology. 1998;508(1):289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lightman S. L., Windle R. J., Wood S. A., Kershaw Y. M., Shanks N., Ingram C. D. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Progress in Brain Research. 2001;133:111–129. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- 158.Windle R. J., Wood S., Shanks N., et al. Endocrine and behavioural responses to noise stress: comparison of virgin and lactating female rats during non-disrupted maternal activity. Journal of Neuroendocrinology. 1997;9(6):407–414. doi: 10.1046/j.1365-2826.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- 159.Neumann I. D. Brain mechanisms underlying emotional alterations in the peripartum period in rats. Depression and Anxiety. 2003;17(3):111–121. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- 160.Pedersen C. A., Ascher J. A., Monroe Y. L., Prange A. J., Jr. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216(4546):648–649. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 161.Gammie S. C., Negron A., Newman S. M., Rhodes J. S. Corticotropin-releasing factor inhibits maternal aggression in mice. Behavioral Neuroscience. 2004;118(4):805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- 162.Bosch O. J., Neumann I. D. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Hormones and Behavior. 2012;61(3):293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 163.de Kloet E. R., Voorhuis D. A. M., Boschma Y., Elands J. Estradiol modulates density of putative ‘oxytocin receptors’ in discrete rat brain regions. Neuroendocrinology. 1986;44(4):415–421. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]
- 164.Bale T. L., Dorsa D. M., Johnston C. A. Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. The Journal of Neuroscience. 1995;15(7 I):5058–5064. doi: 10.1523/JNEUROSCI.15-07-05058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Young L. J., Muns S., Wang Z., Insel T. R. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. Journal of Neuroendocrinology. 1997;9(11):859–865. doi: 10.1046/j.1365-2826.1997.00654.x. [DOI] [PubMed] [Google Scholar]
- 166.Huber D., Veinante P., Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 167.Slattery D. A., Neumann I. D. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. Journal of Physiology. 2008;586(2):377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bosch O. J., Meddle S. L., Beiderbeck D. I., Douglas A. J., Neumann I. D. Brain oxytocin correlates with maternal aggression: Link to anxiety. The Journal of Neuroscience. 2005;25(29):6807–6815. doi: 10.1523/jneurosci.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Francis D. D., Champagne F. C., Meaney M. J. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology. 2000;12(12):1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 170.Grimm S., Pestke K., Feeser M., et al. Early life stress modulates oxytocin effects on limbic system during acute psychosocial stress. Social Cognitive and Affective Neuroscience. 2014;9(11):1828–1835. doi: 10.1093/scan/nsu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.de Souza M. A., Centenaro L. A., Menegotto P. R., et al. Prenatal stress produces social behavior deficits and alters the number of oxytocin and vasopressin neurons in adult rats. Neurochemical Research. 2013;38(7):1479–1489. doi: 10.1007/s11064-013-1049-5. [DOI] [PubMed] [Google Scholar]
- 172.Fan Y., Pestke K., Feeser M., et al. Amygdala-hippocampal connectivity changes during acute psychosocial stress: joint effect of early life stress and oxytocin. Neuropsychopharmacology. 2015;40(12):2736–2744. doi: 10.1038/npp.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Beery A. K., McEwen L. M., MacIsaac J. L., Francis D. D., Kobor M. S. Natural variation in maternal care and cross-tissue patterns of oxytocin receptor gene methylation in rats. Hormones and Behavior. 2016;77:42–52. doi: 10.1016/j.yhbeh.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Unternaehrer E., Meyer A. H., Burkhardt S. C., et al. Childhood maternal care is associated with DNA methylation of the genes for brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) in peripheral blood cells in adult men and women. Stress. 2015;18(4):451–461. doi: 10.3109/10253890.2015.1038992. [DOI] [PubMed] [Google Scholar]
- 175.Belsky J., Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 176.Klengel T., Binder E. B. Gene × environment interactions in the prediction of response to antidepressant treatment. International Journal of Neuropsychopharmacology. 2013;16(3):701–711. doi: 10.1017/s1461145712001459. [DOI] [PubMed] [Google Scholar]
- 177.Finsterwald C., Alberini C. M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiology of Learning and Memory. 2014;112:17–29. doi: 10.1016/j.nlm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aguilera G. HPA axis responsiveness to stress: implications for healthy aging. Experimental Gerontology. 2011;46(2-3):90–95. doi: 10.1016/j.exger.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marshall R. D., Garakani A. Psychobiology of the acute stress response and its relationship to the psychobiology of post-traumatic stress disorder. Psychiatric Clinics of North America. 2002;25(2):385–395. doi: 10.1016/S0193-953X(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 180.Deppermann S., Storchak H., Fallgatter A. J., Ehlis A.-C. Stress-induced neuroplasticity: (mal)adaptation to adverse life events in patients with PTSD—a critical overview. Neuroscience. 2014;283:166–177. doi: 10.1016/j.neuroscience.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 181.Macrì S., Zoratto F., Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neuroscience and Biobehavioral Reviews. 2011;35(7):1534–1543. doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 182.Gee D. G., Humphreys K. L., Flannery J., et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. The Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/jneurosci.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 184.Kim S., Noh D., Park S. I. Mediating effect of stress on the association between early trauma and psychological distress in Korean college students: a cross-sectional observational study. Journal of Psychiatric and Mental Health Nursing. 2015;22(10):784–791. doi: 10.1111/jpm.12262. [DOI] [PubMed] [Google Scholar]
- 185.Nestler E. J., Peña C. J., Kundakovic M., Mitchell A., Akbarian S. Epigenetic basis of mental illness. The Neuroscientist. 2015 doi: 10.1177/1073858415608147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Grundwald N. J., Brunton P. J. Trans-generational effects of prenatal stress on the neuroendocrine stress axis in rats. Psychoneuroendocrinology. 2015;61, article 30 doi: 10.1016/j.psyneuen.2015.07.472. [DOI] [Google Scholar]