Figure 1.
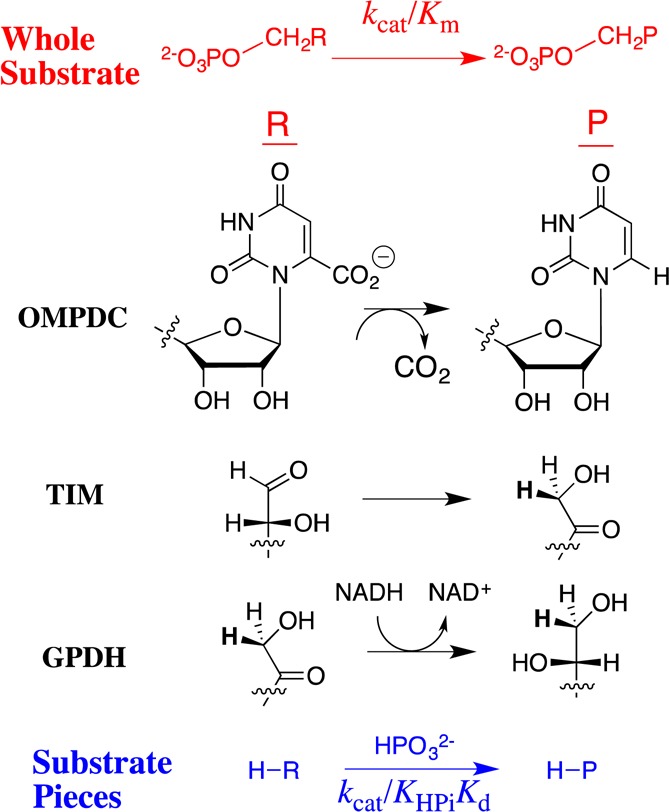
Reactions of whole substrates RCH2OPO32– (kcat/Km) and substrate pieces RH + HPO32– (kcat/KHPiKd) catalyzed by OMPDC, TIM, and GPDH. In each case, 6–8 kcal/mol of the dianion binding energy is used to activate the enzyme for catalysis of the respective reactions of whole and truncated substrates.
