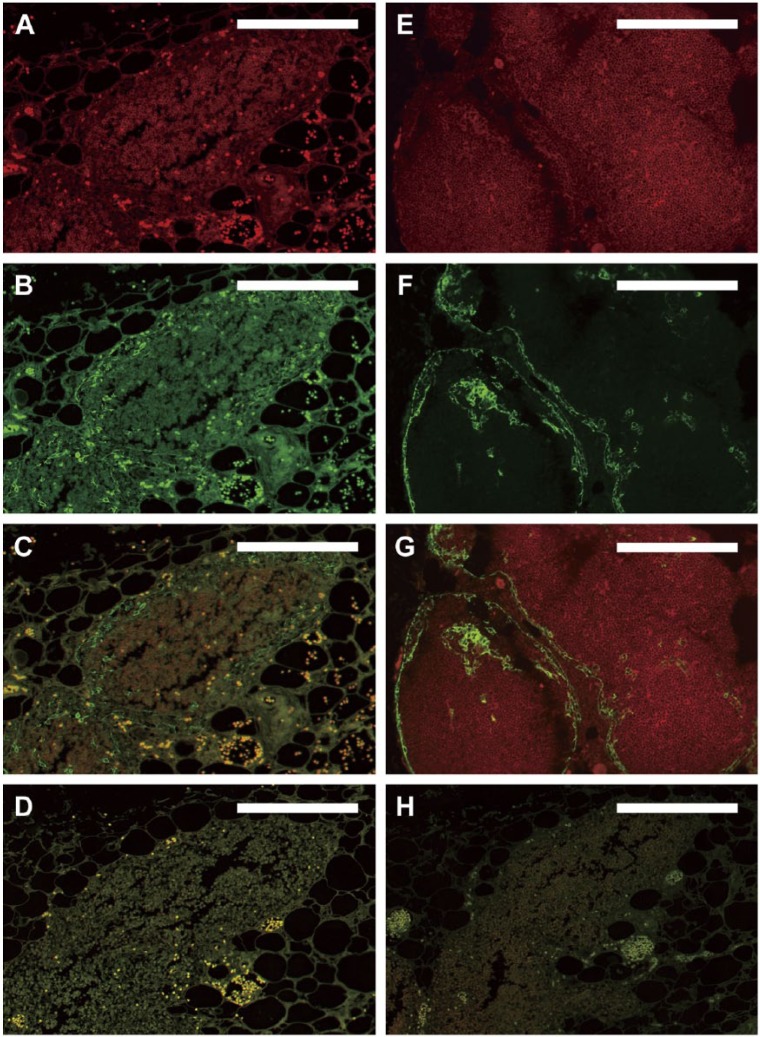
Figure 2.
Immunofluorescence using standard protocols. Dual-color immunofluorescence images were derived from older RERF archival thymus tissues fixed in unbuffered formalin and stored under initially uncontrolled conditions (A–D, H). (A–C) Fluorescence following reaction with anti-CD1a antibody (red, A), anti-CK14 antibody (green, B), and the merged images (C) on a single slide, in the absence of autofluorescence-reducing techniques. A separate section of the same tissue that was deparaffinized but not reacted with antibodies is shown in (D) to document autofluorescence in the absence of background antibody staining. (A–D) High autofluorescence and poor signal-to-noise ratio initially observed with the older archival tissues that prevented definitive identification of cortical thymocytes in (A) and thymic epithelium in (B). For comparison, thymus tissues fixed with neutral-buffered formalin and stored under climate-controlled conditions for 25 years before staining (E–G) show optimal dual-color immunofluorescence images under the same staining conditions used for panels (A–C). Strong specific staining of cortical thymocytes (red, E) and thymic epithelial cells (green, F; merged images in G) is observed in these tissues without the need for additional autofluorescence-reducing techniques. The staining pattern shown in (E–G) thus served as a gold standard for determining optimal IF staining of the older archival tissues from RERF throughout the remainder of the study. (H) Same tissue as in (A–D) with omission of primary antibodies but with application of NH3/EtOH, H2O2, and Sudan Black B treatments, as described in the text. Scale, 0.2 mm.