Figure 2.
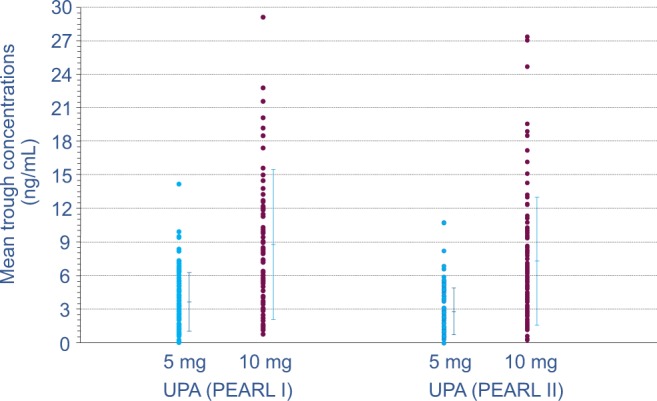
Trough UPA plasma concentrations assessed during patient studies PEARL I24 and PEARL II25. UPA was administered daily at 5 or 10 mg for 3 months to patients with uterine fibroids. Through UPA, plasma concentrations were assessed during weeks 5, 9, and 13 of treatment. Individual (•) and mean values (with standard deviation) are plotted. PEARL indicates PGL4001’s Efficacy Assessment in Reduction of symptoms due to uterine Leiomyomata. UPA indicates ulipristal acetate.
