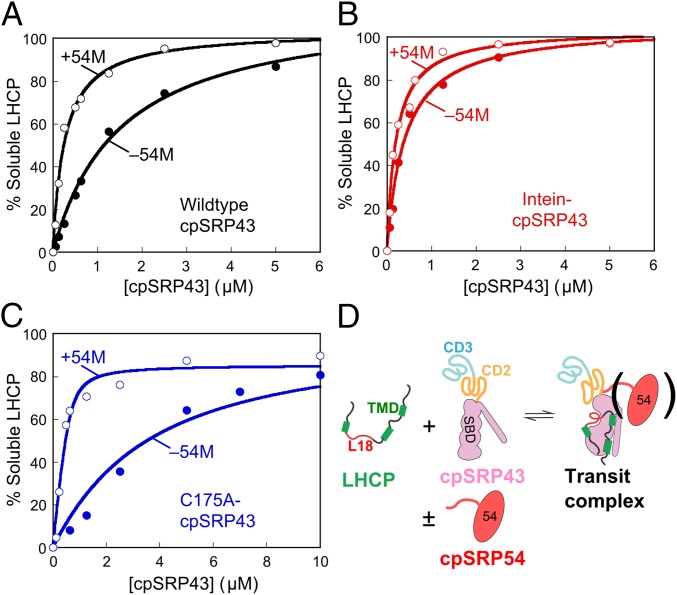
Fig. 3.
The cpSPR54 M-domain activates cpSRP43 for substrate binding. The abilities of chaperone to prevent LHCP aggregation were measured for WT cpSRP43 (A), superactive intein-cpSRP43 (B), and partially defective mutant cpSRP43(C175A) (C) in the absence (●) and presence (○) of 54M. The data were fit to a Michaelis–Menton equation and gave apparent Kd values of 0.26 and 1.5 µM for WT cpSRP43 with and without 54M (A), 0.20 and 0.41 µM for intein-cpSRP43 with and without 54M (B), and 0.08 and 3.0 µM for cpSRP43(C175A) with and without 54M (C). In A and B, activities were measured under 100 mM NaCl, a stringent condition under which cpSRP43 exhibits slightly reduced activity, to overcome the saturation effects with highly active chaperone constructs and better reveal the stimulatory effects of 54M. (D) Scheme depicting the conformational change of cpSRP43 on substrate binding. TMD, transmembrane domain.