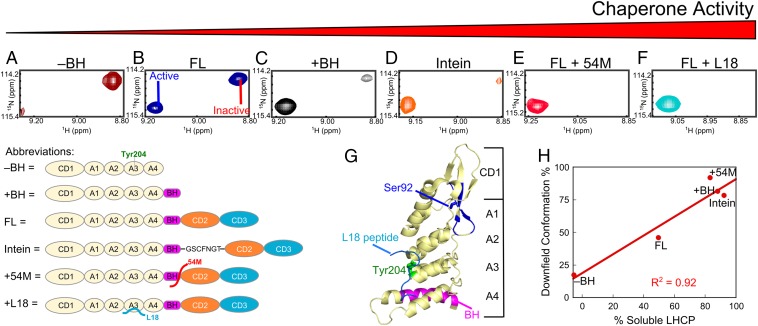
Fig. 4.
Conformational dynamics of cpSRP43 correlates with its chaperone activity. (A–F) Component cross-peaks for Ser92 in the TROSY spectrum of 2H,15N-labeled CD1Ank fragment (A), full-length cpSRP43 (B), CD1Ank-BH fragment (C), intein-cpSRP43 (D), cpSRP43 bound to 54M peptide (E), and cpSRP43 bound to the L18 peptide (F). (G) Crystal structure of CD1Ank-BH fragment (PDB ID code 3DEP) highlighting the residues in CD1 (blue) for which component cross peaks reflecting the conformational dynamics of cpSRP43 have been unambiguously assigned. Green shows Tyr204 in Ank3, which binds L18 (cyan). Magenta highlights the bridging helix. (H) Correlation between the chaperone activity (% soluble LHCP observed with 0.625 µM chaperone) of each construct or complex and the relative intensity of the downfield component cross-peak.