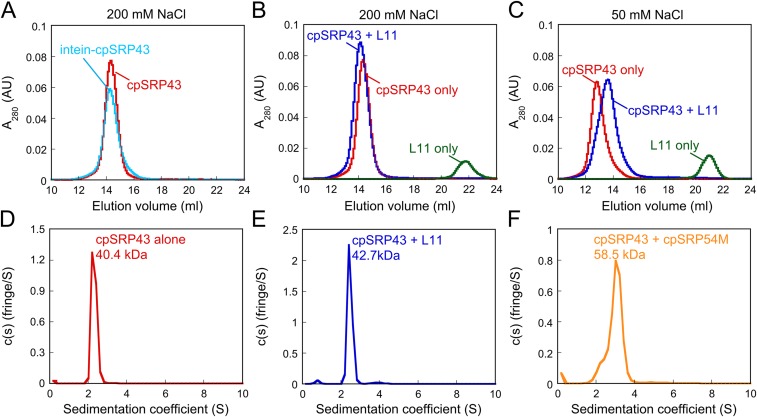
Fig. S2.
CpSRP43 is active as a monomer. (A) WT (red) and superactive intein-cpSRP43 (cyan) runs as a monomer on Superdex 200 column with buffer containing 200 mM NaCl. (B) The complex of WT cpSRP43 and HiLyte-Fluor488–labeled L11 peptide (blue) was eluted as a 1:1 complex. (C) At lower ionic strength (50 mM NaCl), cpSRP43 exhibits oligomeric forms (red), but L11 binding shifts cpSRP43 to a lower molecular weight complex (blue). (D–F) Sedimentation coefficient distributions calculated from a velocity sedimentation experiment of cpSRP43 alone (D), cpSRP43 with HiLyte-Fluor488–labeled L11 peptide (E), and cpSRP43 with cpSRP54M (F) using buffer containing 200 mM NaCl. The experimental molecular mass is close to the predicted values of cpSRP43 and cpSRP54M (36 and 22 kDa, respectively), suggesting that the active form of cpSRP43 is monomer.