Significance
Three-dimensional systems enable the formation of tissue-mimetic architectures and promote more realistic physiological responses than conventional 2D systems. Here we report a previously unidentified layered 3D culture system to assay migration and maturation of human induced pluripotent stem cell (iPSC)-derived neural progenitor cells (NPCs) and reveal a genotype-specific effect of methyl-CpG-binding protein-2 (MeCP2) dysfunction on iPSC-derived neuronal migration and maturation in 3D layered hydrogels. Using this platform, we identified a migration defect in MeCP2-mutant iPSC-derived NPCs and confirmed previous observations that neurons derived from these cells have reduced neurite outgrowth and fewer synapses. Meanwhile, 3D hydrogel culture accelerates neuronal differentiation of iPSC-derived NPCs.
Keywords: 3D hydrogels, neuronal migration and maturation, 3D RTT modeling
Abstract
Probing a wide range of cellular phenotypes in neurodevelopmental disorders using patient-derived neural progenitor cells (NPCs) can be facilitated by 3D assays, as 2D systems cannot entirely recapitulate the arrangement of cells in the brain. Here, we developed a previously unidentified 3D migration and differentiation assay in layered hydrogels to examine how these processes are affected in neurodevelopmental disorders, such as Rett syndrome. Our soft 3D system mimics the brain environment and accelerates maturation of neurons from human induced pluripotent stem cell (iPSC)-derived NPCs, yielding electrophysiologically active neurons within just 3 wk. Using this platform, we revealed a genotype-specific effect of methyl-CpG-binding protein-2 (MeCP2) dysfunction on iPSC-derived neuronal migration and maturation (reduced neurite outgrowth and fewer synapses) in 3D layered hydrogels. Thus, this 3D system expands the range of neural phenotypes that can be studied in vitro to include those influenced by physical and mechanical stimuli or requiring specific arrangements of multiple cell types.
Neuronal migration and maturation is a key step in brain development. Defects in this process have been implicated in many disorders, including autism (1) and schizophrenia (2). Thoroughly understanding how neural progenitor cell (NPC) migration is affected in neurodevelopmental disorders requires a means of dissecting the process using cells with genetic alterations matching those in patients. Existing in vitro assays of migration generally involve measurement of cell movement across a scratch or gap or through a membrane toward a chemoattractant in 2D culture systems. Although widely used, such assays may not accurately reveal in vivo differences, as neuronal migration is tightly regulated by physical and chemical cues in the extracellular matrix (ECM) that NPCs encounter as they migrate.
In vitro 3D culture systems offer a solution to these limitations (3–7). Compared with 2D culture, a 3D arrangement allows neuronal cells to interact with many more cells (4); this similarity to the in vivo setting has been shown to lengthen viability, enhance survival, and allow formation of longer neurites and more dense networks in primary neurons in uniform matrices or aggregate culture (8, 9). Indeed, 3D culture systems have been used to study nerve regeneration, neuronal and glial development (10–12), and amyloid-β and tau pathology (13). Thus, measuring neuronal migration through a soft 3D matrix would continue this trend toward using 3D systems to study neuronal development and pathology.
We sought to develop a 3D assay to examine potential migration and neuronal maturation defects in Rett syndrome (RTT), a genetic neurodevelopmental disorder that affects 1 in 10,000 children in the United States and is caused by mutations in the X-linked methyl-CpG-binding protein-2 (MECP2) gene (14). Studies using induced pluripotent stem cells (iPSCs) from RTT patients in traditional 2D adherent culture have revealed reduced neurite outgrowth and synapse number, as well as altered calcium transients and spontaneous postsynaptic currents (1). However, 2D migration assays seemed unlikely to reveal inherent defects in this developmental process, which could be affected because MeCP2 regulates multiple developmental related genes (15). Migration of RTT iPSC-derived NPCs has not previously been studied.
Using a previously unidentified 3D tissue culture system that allows creation of layered architectures, we studied differences in migration of MeCP2-mutant iPSC-derived versus control iPSC-derived NPCs. This approach revealed a defect in migration of MeCP2-mutant iPSC-derived NPCs induced by either astrocytes or neurons. Further, this 3D system accelerated maturation of neurons from human iPSC-derived NPCs, yielding electrophysiologically active neurons within just 3 wk. With mature neurons derived from RTT patients and controls, we further confirmed defective neurite outgrowth and synaptogenesis in MeCP2-mutant neurons. Thus, this 3D system enables study of morphological features accessible in 2D system as well as previously unexamined phenotypes.
Results
Modular Design of Layered Hydrogels for Migration of Human iPSC-Derived NPCs.
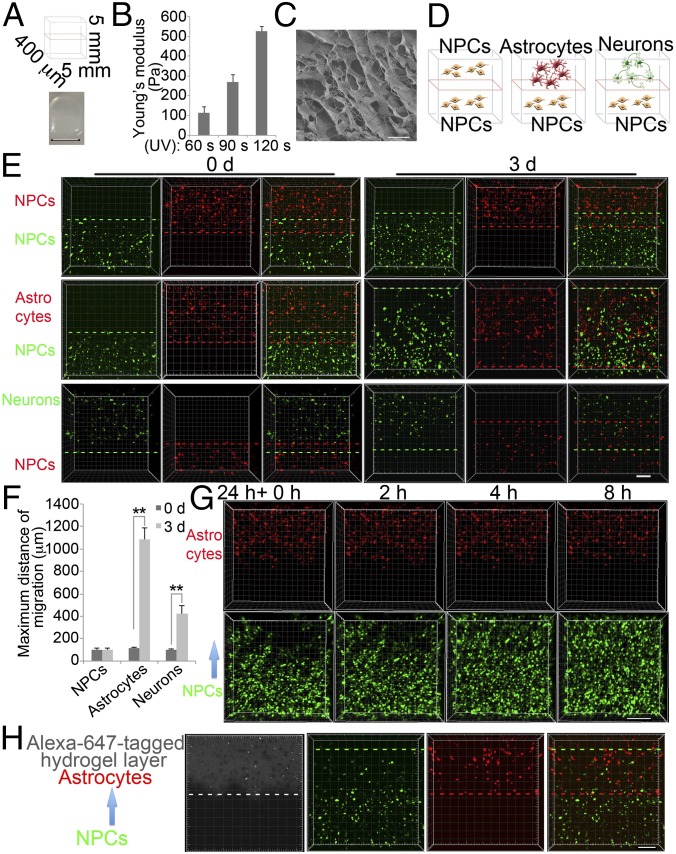
We developed a previously unidentified and simple assay measuring migration through a soft 3D matrix to better imitate the physical environment within the brain. This 3D hydrogel-based migration assay relies on density gradient multilayer polymerization (16), which consists simply of mixing small-molecule density modifiers with prepolymer-containing cell suspensions and gently layering them with a syringe. The prepolymer in these studies was methacrylate-modified hyaluronic acid (HAMA, 17 ± 1% methacrylation); varying ultraviolet A (UVA) irradiation time yielded hydrogels of variable stiffness (Fig. 1 A and B). Hydrogels with stiffness around 100 Pa and pore size around 10 µm were used (Fig. 1C). By recreating cell−cell interactions, 3D layered structures enable the formation of tissue-mimetic architectures and promote more realistic physiological responses than conventional 2D culture. We used the layered hydrogel model to investigate the migration of NPCs toward different neuronal cell types: NPCs, astrocytes, or neurons (Fig. 1D). As astrocytes have been shown to promote neural migration (17), we first evaluated NPC migration toward these cells. Human NPCs were derived from human nonaffected control iPSC lines (WT83) and infected with lentivirus expressing green fluorescent protein (GFP) under the control of the cytomegalovirus (CMV) promoter to track migration by microscopy. Immunofluorescence and flow cytometry confirmed expression of progenitor markers Nestin, Sox1, Sox2, and PAX6 (Fig. S1 A and B). Human astrocytes were generated from differentiated human NPCs and infected with lentivirus expressing tdTomato under the control of the glial fibrillary acidic protein (GFAP) promoter, then enriched by flow cytometry for tdTomato-positive cells (Fig. S1 C and D). Immunofluorescence confirmed expression of astrocyte markers, GFAP, and S100 calcium-binding protein β (S100β) (Fig. S1E). Migration was examined in two-layered hydrogels containing sorted GFAP::tdTomato-positive astrocytes in the top and CMV::GFP-positive NPCs in the bottom layer, cultured in either neuronal growth medium (NG) or astrocyte culture medium (AG), respectively. After 3 d, we observed dramatic NPC migration toward astrocytes (Fig. 1 E and F). Using real-time fluorescence microscopy, we showed NPC migration toward astrocytes in a time-dependent manner (Fig. 1G). To further confirm that NPCs in the bottom layer were migrating toward astrocytes, we used fluorescence-labeled HAMA to identify the top layer in our 3D migration assay. We found that GFAP::tdTomato-positive astrocytes remained in the fluorescence-labeled top layer, whereas CMV::GFP-positive differentiating NPCs migrated into it (Fig. 1H).
Fig. 1.
Setup of layered hydrogels to study migration of human iPSC-derived NPCs. (A) Bright-field image of two-layered hydrogels. (Scale bar, 5 mm.) (B) Compressive moduli of HAMA hydrogels measured by atomic force microscope (AFM) following varying UV exposure time. (C) Scanning electron microscopy (SEM) imaging of HAMA hydrogels. (Scale bar, 10 µm.) (D) Schematic representation of a two-layered hydrogel assay of NPC migration toward NPCs, astrocytes, or neurons. (E) Representative images of NPC migration induced by NPCs, astrocytes, or neurons for indicated times. Dotted lines identify the farthest cell of each type; the distance between red and green dotted lines was considered the maximum migration distance. (Scale bar, 200 μm.) (F) Quantification of maximum migration distance induced by NPCs, astrocytes, or neurons (see Materials and Methods). Bars represent means; *P < 0.05, and **P < 0.01; n = 3. (G) Time-lapse images of NPC migration induced by astrocytes for 8 h. (Scale bar, 200 μm.) (H) Representative images of NPC migration induced by astrocytes for 1.5 d. Alexa647-labeled HAMA identifies the top layer. (Scale bar, 200 μm.)
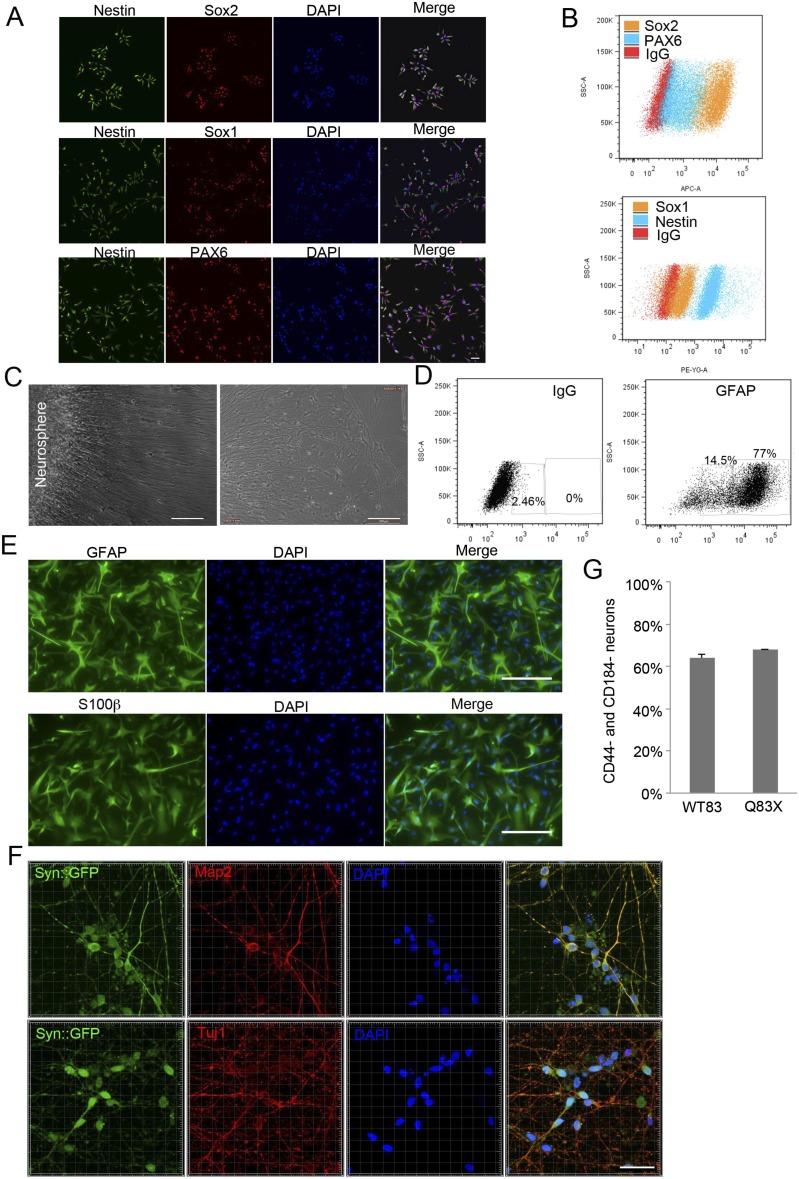
Fig. S1.
Characterization of NPCs derived from human iPSCs and astrocytes and neurons from human NPCs. (A) Representative immunofluorescence of WT83 NPCs stained for Nestin, Sox2, Sox1, PAX6, and DAPI. (Scale bar, 50 μm.) (B) FACS analysis of PAX6, Sox2, Nestin, and Sox1 expression in WT83 NPCs. (C) Representative images of NPC-derived astrocytes migrating from a neurosphere. (Scale bar, 200 μm.) (D) FACS analysis of GFAP expression in NPC-derived astrocytes. (E) Representative image of NPC-derived astrocytes immunostained for GFAP and S100β. (Scale bar, 100 μm.) (F) Representative image of NPC-derived neurons in 2D immunostained for Tuj1 and Map2 (red), expressed Syn::GFP (green), and stained with DAPI (blue). (Scale bar, 30 μm.) (G) Magnetic-assisted cell sorting analysis of NPC-derived CD44- and CD184-negative neurons.
We further evaluated NPC migration induced by neurons in our 3D system. Human neurons positive for the neuronal markers β-III-tubulin (Tuj1) and Map2 (Fig. S1F) were generated from differentiated human NPCs as described (1) and enriched by magnetic-activated cell sorting for CD44 and CD184 double-negative cells (Fig. S1G). CD44 and CD184 were cell-surface markers for the isolation of neurons derived from human pluripotent stem cells (18). These neurons were seeded in the top layer, and CMV::tdTomato NPCs were seeded in the bottom layer. We observed NPC migration induced by neurons after 3 d of coculture (Fig. 1 E and F). On the contrary, when NPCs were seeded in both layers, NPCs in the bottom layer did not migrate (Fig. 1 E and F). Taken together, this 3D assay measures NPC migration toward either astrocytes or neurons.
Defective Migration of NPCs Derived from RTT iPSCs.
With this 3D migration system, we sought to examine whether MeCP2 mutations found in RTT patients affect neuronal migration, as MeCP2 regulates multiple genes involved in this process (15). Further, mutations in one of these, cyclin-dependent kinase-like 5 (CDKL5), have been identified in patients with RTT (19); knockdown of CDKL5 in rats causes delayed neuronal migration (20). Despite the current consensus that RTT does not involve migration defects (21), this process may be affected in some RTT-affected individuals.
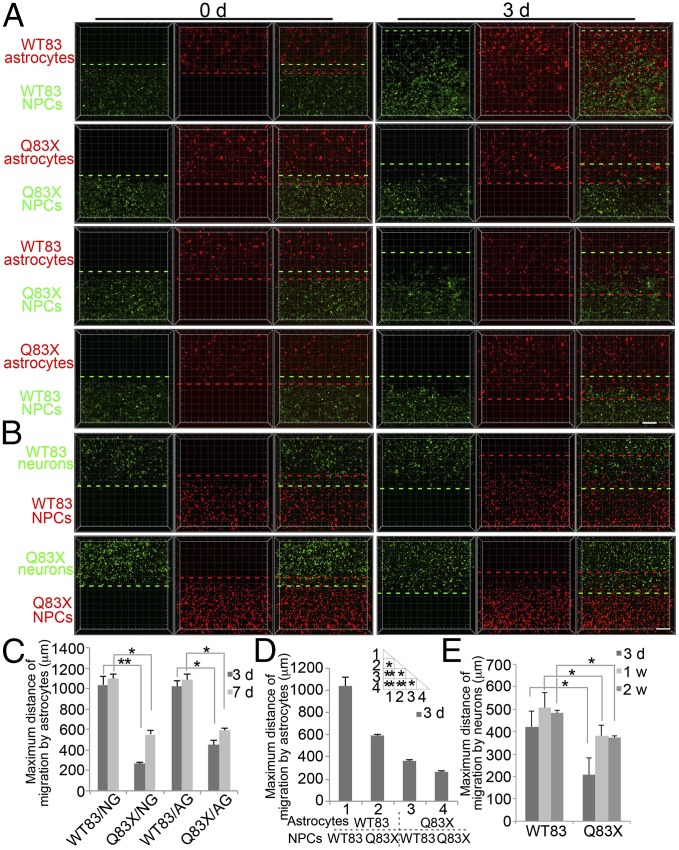
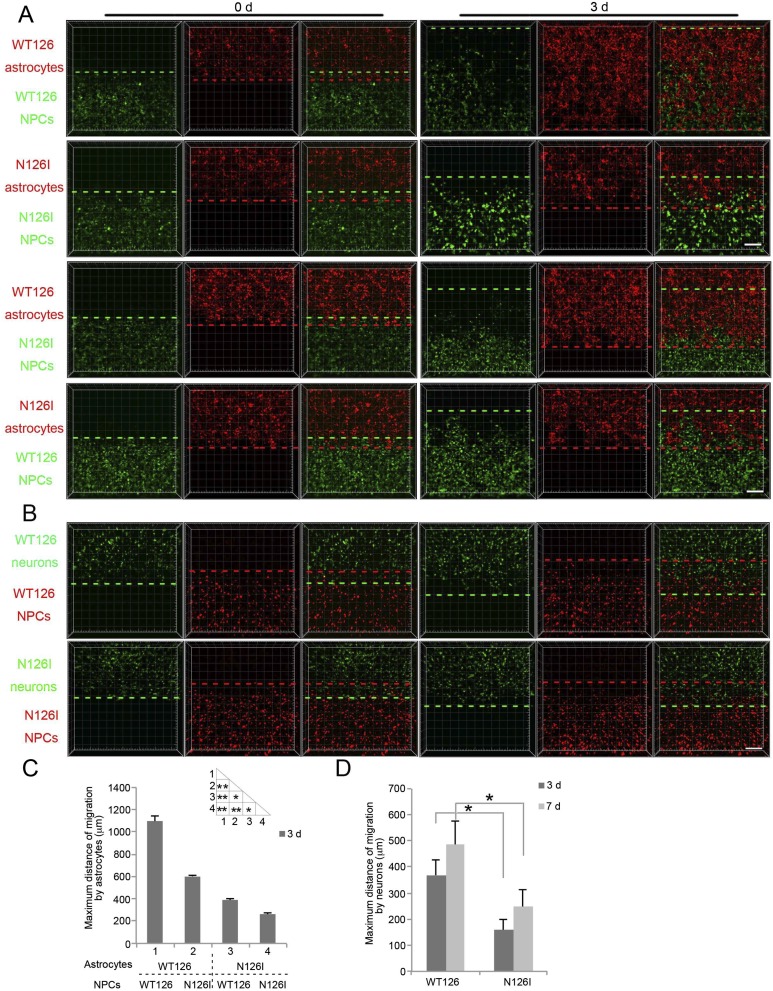
We compared migration of NPCs derived from RTT patients and their parental controls (1). First, we used NPCs derived from a male RTT patient (Q83X) and his nonaffected father’s iPSC lines. Migration was examined in two-layer hydrogels containing sorted GFAP::tdTomato-positive astrocytes in the top layer and CMV::GFP-positive NPCs in the bottom layer. Q83X NPC migration toward Q83X astrocytes was retarded ∼70% compared with WT83 NPCs toward WT83 astrocytes, indicating a migration defect (Fig. 2 A and C). In the meantime, Q83X NPCs migration toward WT83 astrocytes was still retarded ∼40% comparing to WT83 NPCs migration toward WT83 astrocytes, indicating intrinsic defect of Q83X NPCs migration itself, whereas WT83 NPC migration toward Q83X astrocytes was also ∼55% retarded comparing to WT83 NPC migration toward WT83 astrocytes, indicating defect of Q83X astrocytes chemoattraction (Fig. 2 A and D). Thus, the phenotype appears to reflect both slower intrinsic migratory ability of Q83X NPCs and impaired chemoattraction by Q83X astrocytes. We further evaluated WT83 and Q83X NPC migration induced by neurons. Q83X NPC migration toward neurons was also retarded compared with WT83 NPCs (Fig. 2 B and E). Taken together, RTT NPCs carrying the Q83X variant of MeCP2 migrate shorter distances toward either astrocytes or neurons.
Fig. 2.
Defective migration of Q83X NPCs toward astrocytes or neurons. (A) Representative microscopy images of WT83 and Q83X NPC migration induced by WT83 or Q83X astrocytes for indicated times. (Scale bar, 200 μm.) (B) Representative microscopy images of WT83 and Q83X NPC migration toward neurons for indicated times. (Scale bar, 200 μm.) (C) Quantification of maximum WT83 or Q83X NPC migration distance induced by WT83 or Q83X astrocytes in NG and AG medium (see Materials and Methods). Bars represent means; *P < 0.05, and **P < 0.01; n = 3. (D) Quantification of maximum WT83 NPC migration distance induced by WT83 or Q83X astrocytes and maximum Q83X NPC migration distance induced by WT83 or Q83X astrocytes in AG medium. Bars represent means; *P < 0.05, and **P < 0.01; n = 3. (E) Quantification of maximum migration distance toward neurons. Bars represent means; *P < 0.05; n = 3.
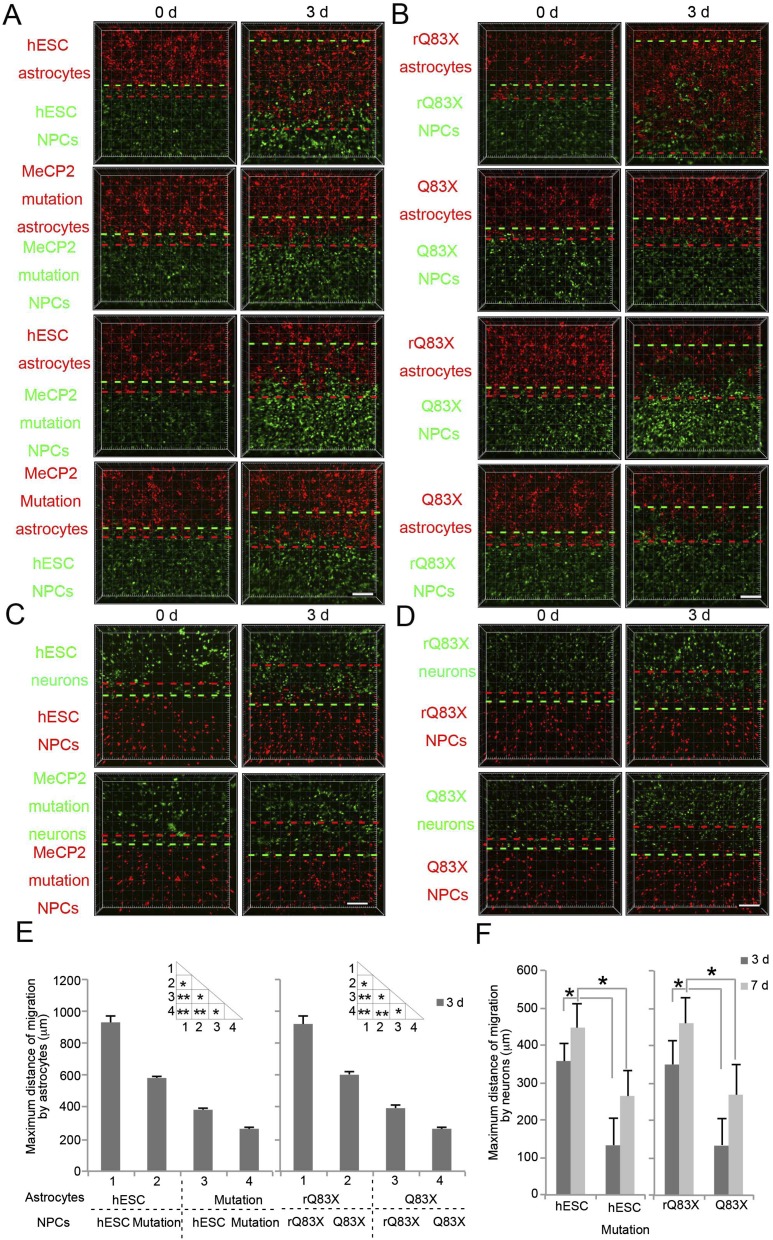
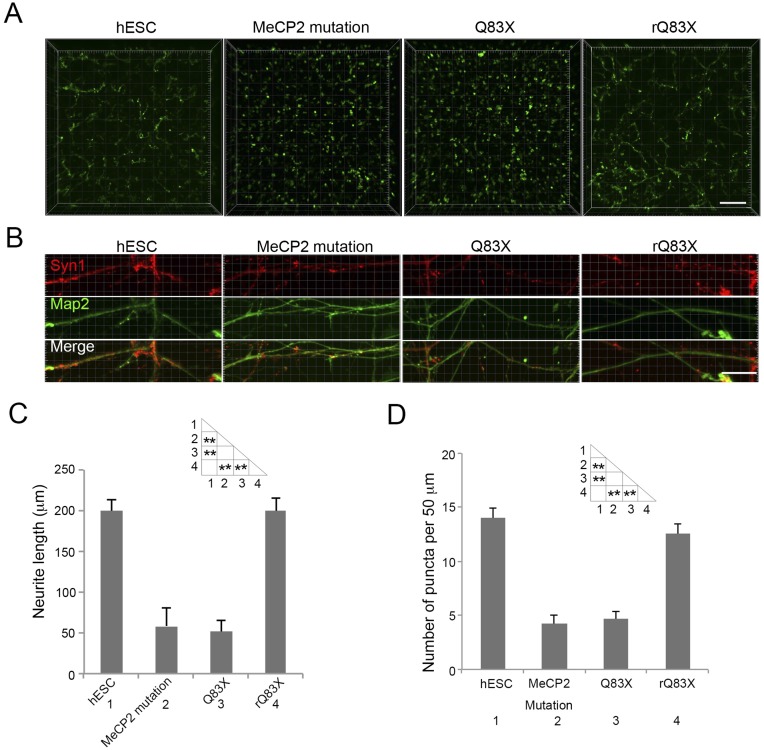
To rule out the influence of genetic factors besides the MeCP2 Q83X mutation as a cause of this phenotype, we used isogenic stem cells carrying a frame-shift mutation in the MECP2 gene in a human embryonic stem cell (hESC) line (hESC–MeCP2 mutation). Similar migration assays using NPCs derived from these cells validated the defect in migration toward both astrocytes (Fig. S2 A and E) and neurons (Fig. S2 C and F). Additionally, we rescued a patient cell line with an early stop codon mutation (Q83X), restoring MeCP2 protein levels to normal (rQ83X). The defects in migration toward both astrocytes (Fig. S2 B and E) and neurons (Fig. S2 D and F) were rescued when MeCP2 was restored in the cells (rQ83X). These results suggest that MeCP2 mutations impair cell-induced migration in 3D hydrogels.
Fig. S2.
Isogenic cell-derived NPCs mimic migration defect of Q83X NPCs. (A) Representative images of hESC or MeCP2-mutated hESC (hESC–MeCP2 mutation)-derived NPC migration toward astrocytes. (Scale bar, 100 μm.) (B) Representative images of Q83X or patient rescued Q83X (rQ83X)-derived NPC migration toward astrocytes. (Scale bar, 100 μm.) (C) Representative images of hESC or hESC–MeCP2 mutation-derived NPC migration toward neurons. (Scale bar, 100 μm.) (D) Representative images of Q83X or rQ83X-derived NPC migration toward neurons. (Scale bar, 100 μm.) (E) Quantification of maximum migration distance toward astrocytes (see Materials and Methods). Bars represent means; *P < 0.05, and **P < 0.01; n = 3. (F) Quantification of maximum migration distance toward neurons. *P < 0.05; n = 3.
To further confirm the migration defects caused by dysfunction of MeCP2, we repeated the 3D migration assay with NPCs derived from another male RTT patient (N126I) and his nonaffected father’s iPSC lines. Migration was examined in two-layer hydrogels containing sorted GFAP::tdTomato-positive astrocytes in the top layer and CMV::GFP-positive NPCs in the bottom layer. Similar migration defect due to both slower intrinsic migratory ability of N126I NPCs and impaired chemoattraction by N126I astrocytes was found (Fig. S3 A and C). We further evaluated WT126 and N126I NPC migration induced by neurons. N126I NPC migration toward neurons was also retarded compared with WT126 NPCs (Fig. S3 B and D). Taken together, RTT NPCs carrying the N126I variant of MeCP2 mutation migrate shorter distances toward either astrocytes or neurons.
Fig. S3.
Defective migration of N126I NPCs toward astrocytes or neurons. (A) Representative images of WT126 and N126I NPC migration induced by astrocytes for indicated times. (Scale bar, 200 μm.) (B) Representative images of WT126 and N126I NPC migration toward neurons for indicated times. (Scale bar, 200 μm.) (C) Quantification of maximum migration distance induced by astrocytes (see Materials and Methods). Bars represent means; *P < 0.05, and **P < 0.01; n = 3. (D) Quantification of maximum migration distance toward neurons. Bars represent means; *P < 0.05; n = 3.
Three-Dimensional Hydrogel Culture Accelerates Neuronal Differentiation of Human iPSC-Derived NPCs.
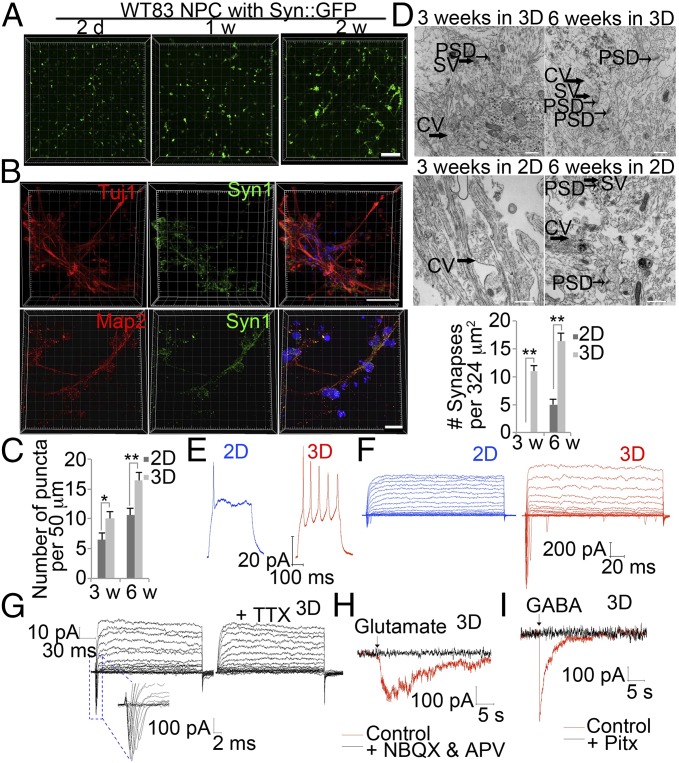
As an initial step toward investigating RTT pathophysiology, we generated mature neurons from human iPSC-derived NPCs in our 3D culture system. NPCs were infected with lentivirus expressing GFP from the neuron-specific synapsin-1 (Syn) promoter, and differentiation was followed by monitoring GFP fluorescence. Control iPSC-derived Syn::GFP NPCs (WT83) expressing Nestin, Sox2, Sox1, and PAX6 (Fig. S1 A and B) were seeded in 3D hydrogels. Expression of GFP, indicating neuronal differentiation, was detectable within only 2 d (Fig. 3A). After 1 wk of culture in the hydrogel, neurite outgrowth from NPCs was already clearly visible, and neurites > 100 µm were apparent throughout the thickness of the hydrogel by week two (Fig. 3A). These cells were positive for neuronal markers such as Tuj1 and microtubule-associated protein 2 (Map2). Moreover, we also observed synapsin puncta outlining Map2-positive neurites (Fig. 3B). Compared with 2D culture, the density of syn1-positive puncta in 3D culture was significantly higher after 3 wk culture and was even more significant after 6 wk culture (Fig. 3C). To further confirm formation of synapses, we examined ultrastructure. Electron microscopy revealed typical synaptic vesicles and postsynaptic densities after 3 wk of differentiation in 3D hydrogels but only large clear vesicles at the same time in 2D culture. Only after 6 wk of differentiation in 2D culture did the postsynaptic densities appear. The density of synapses was significantly higher in 3D culture compared with 2D culture after both 3 wk and 6 wk of differentiation (Fig. 3D).
Fig. 3.
Rapid maturation of human iPSC-derived neurons in 3D hydrogels. (A) Representative images of human iPSC-derived NPCs infected with Syn::GFP lentivirus after neuronal differentiation for indicated times in 3D hydrogels. (Scale bar, 200 μm.) (B) Representative images of human iPSC-derived neurons stained for Tuj1 or Map2 (red), Syn1 (green), and DAPI (blue) in 3D hydrogel. (Scale bar, 60 μm.) (C) Quantification of Syn1 puncta on Map2 neurites. Bars represent means of 50 neurons per condition; *P < 0.05, and **P < 0.01; n = 3. (D) Representative images of ultrastructural investigation of synaptogenesis by transmission electron microscopy in control NPCs differentiated in 3D or 2D system at indicated times. CV, large clear vesicle; SV, synaptic vesicle. Quantification of numbers of synapses in control NPCs differentiated in 3D or 2D system at indicated times. Bars represent means; **P < 0.01 by; n = 3. (Scale bar, 500 nm.) (E) Representative whole-cell current clamp recordings of human iPSC-derived neurons after 3 wk differentiation in 3D or 2D system. Spiking activity was examined following current injection. (F) Representative voltage clamp recording of a 3D or 2D cultured neuron held at −75 mV and then stepped to a series of voltages (−80 to +20 mV) in 5-mV increments. (G) Representative voltage clamp recording of a 3D cultured neuron before and after application of TTX. TTX, 1 μM tetrodotoxin. (H) Response to glutamate in the presence and absence of the glutamate receptor blockers 20 μM NBQX and 50 μM APV. (I) Response to GABA in the presence and absence of 50 μM picrotoxin (Pitx).
To examine whether 3D differentiated neurons were functionally mature, we transferred them to glass slides by digesting hydrogels containing NPCs expressing Syn::GFP and differentiated for 3 wk using hyaluronidase (2,000 units per milliliter) overnight. After 4 d of culture on slides, electrophysiological activity of GFP-positive cells was examined. Predigestion of 3D hydrogel is for recording and comparison with 2D differentiated neurons. The 2D differentiated neurons were also treated with hyaluronidase. In response to steps of depolarizing current, only neurons differentiated in 3D hydrogels but not in 2D culture for 3 wk fired trains of action potentials (Fig. 3E). Further, whole-cell voltage clamp recordings performed before and after application of 1 μM tetrodotoxin revealed functional voltage-activated sodium and potassium channels in 3D differentiated cells, but not in 2D culture, indicating fast maturation of 3D cultured cells (Fig. 3 F and G). In addition, we detected whether 3D differentiated neurons expressed functional glutamate and GABA receptors. Bath application of the neurotransmitter glutamate (100 μM) transiently induced an inward current, and these events were blocked by 20 μM 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) and 50 μM d-aminophosphonovalerate (APV), indicating the presence of functional glutamate receptors (Fig. 3H). Transient currents were also observed following 10 μM γ-aminobutyric acid (GABA) bath application, indicating the presence of functional GABA receptors, as these events were blocked by 50 μM picrotoxin (Fig. 3I). All these results suggest that NPC-derived neurons differentiated in 3D hydrogels for only 3 wk are functionally mature.
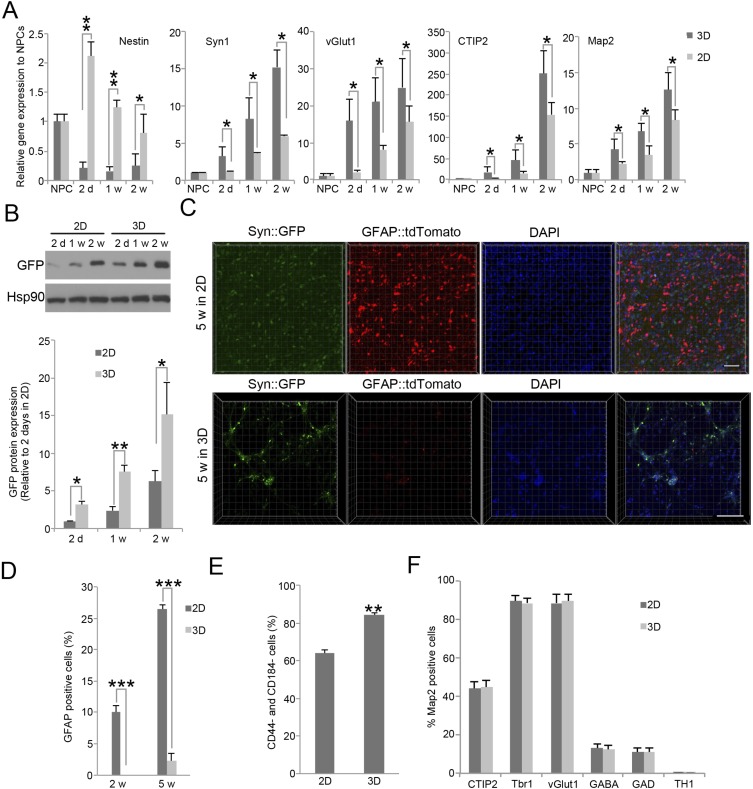
We further compared neuronal differentiation of Syn::GFP-expressing NPCs cultured either in 2D adherent culture or 3D hydrogels. By real-time PCR, the NPC marker Nestin was dramatically down-regulated in 3D cultured but not in 2D cultured cells, whereas neuronal markers, such as Syn1, vGlut1, CTIP2, and Map2 were elevated in both. However, mRNA expression of these neuronal markers in 3D cultured cells was significantly greater than that in 2D culture (Fig. S4A). Consistently, Syn promoter-driven GFP expression in 3D culture was also higher than in 2D culture (Fig. S4B), indicating greater neuronal differentiation.
Fig. S4.
Neuronal differentiation in 3D versus in 2D system. (A) The mRNA expression of NPC (Nestin) and neuronal marker genes (Syn1, vGlut1, CTIP2, and Map2) in cells cultured either in 3D or in 2D system. Bars represent means; *P < 0.05, and **P < 0.01; n = 3. (B) Syn1 promoter-driven GFP protein expression after differentiation in 3D or 2D system. (C) Representative images of cells infected with lentivirus expressing both GFAP::tdTomato and Syn::GFP after neuronal differentiation at 5 wk in 3D or 2D system. (D) Quantification of GFAP-positive cells after differentiation in 3D or 2D system. Bars represent means; ***P < 0.005; n = 3. (E) Quantification of CD44 and CD184 double-negative cells after differentiation in 3D or 2D system. Bars represent means; **P < 0.01; n = 3. (F) Quantification of inhibitory (GABA+, GAD1+), excitatory (vGlut1+, Tbr1+) neurons, layer V/VI (CTIP2+) neurons, and dopaminergic (TH1+) neurons in Map2-positive neurons. Bars represent means; n = 3.
To examine the proportion of NPCs that differentiate into neurons vs. astrocytes in 3D and 2D culture, we infected iPSC-derived NPCs with both lentiviral Syn::GFP and GFAP::tdTomato (GFAP is an astrocyte marker). Although, in 2D culture, 10% of cells were GFAP-positive at 2 wk differentiation, few GFAP-positive cells were detected in 3D culture at this time point. At 5 wk, 2D cultures contained 26% GFAP-positive cells versus only 3.5% in 3D culture (Fig. S4 C and D). Further, a greater proportion of 3D than 2D cultured cells were double-negative for CD44 and CD184 (85% vs. 63%; Fig. S4E), cell surface markers used to isolate neurons derived from human pluripotent stem cells (18). These results show that differentiation of iPSC-derived NPCs in 3D hydrogels favors neuronal over glial differentiation and accelerates maturation of neurons compared with 2D culture.
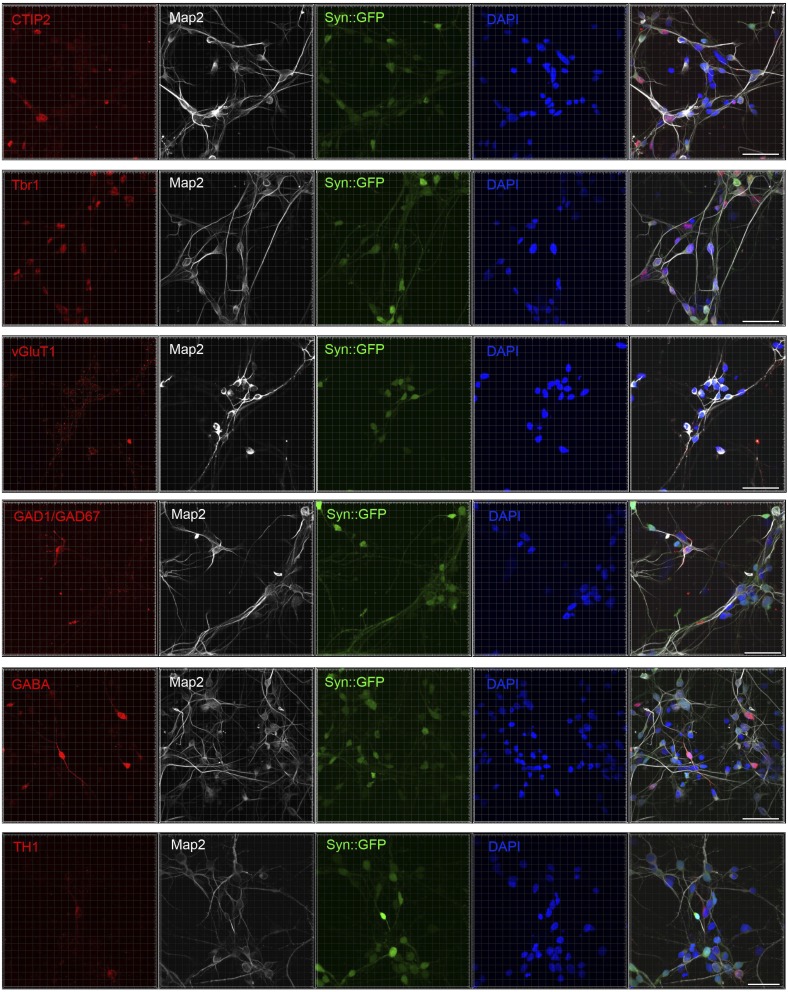
Next, we directly tested whether there was any bias for differentiation into specific neuronal subtypes. In our 3D system, neurons were cultured in NG medium, which is widely used for differentiation of human cortical neurons (1, 22). Thus, we used TBR1 and vGluT1 to determine the proportions of cortical excitatory neurons, GABA and GAD1/GAD67 (the GABA synthetic enzyme) for GABAergic inhibitory neurons, CTIP2 (also known as BCL11B) for layer V/VI neuron, and TH1 for dopaminergic neuron (23) (Fig. S5). Most importantly, there was no significant difference in subtype populations between 3D cultured and 2D cultured neurons (Fig. S4F).
Fig. S5.
Representative image of WT83 NPC-derived neurons in 3D hydrogel immunostained for CTIP2, Tbr1, vGlut1, GAD1, GABA, and TH1 (red), and Map2 (gray), expressed Syn::GFP (green), and stained with DAPI (blue). (Scale bar, 50 μm.)
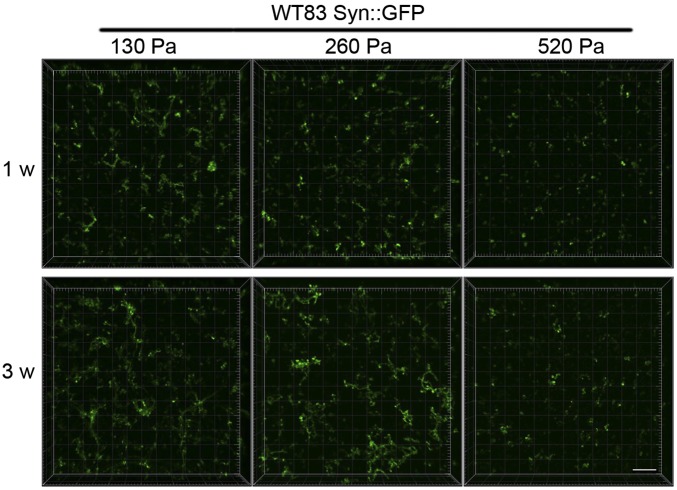
Hydrogel scaffold mechanical properties and presentation of biochemical cues can simulate the in vivo environment of the brain (24). Most importantly, the defined elastic modulus range of our HAMA hydrogels is consistent with native neural microenvironments (50–250 Pa), which is important for directing neural survival and axonal outgrowth (25, 26). We carefully tuned the mechanics of our system to accurately represent the in vivo condition. For example, we expected that scaffold elasticity would influence the extent of neurite outgrowth for differentiating NPCs. We tested our hypothesis by tracking extension length as a function of varied elastic moduli over a biologically relevant range. As shown in Fig. 1B, altering the UVA irradiation time at 60 s, 90 s, or 120 s resulted in HAMA hydrogel matrices with elastic moduli of 130 Pa, 260 Pa, and 520 Pa, respectively. Neurons differentiated within more elastic 130-Pa scaffolds exhibited greater neurite outgrowth compared with those differentiated within less elastic, 260 Pa and 520 Pa, scaffolds (Fig. S6). These data illustrate our effort to identify and optimize scaffold conditions critical to our 3D neural model of migration, differentiation, and neurite outgrowth.
Fig. S6.
Effects of hydrogel mechanical properties on differentiation and neurite outgrowth of NPCs in 3D. Representative images of cells infected with lentivirus expressing Syn::GFP after neuronal differentiation at 1 wk and 3 wk in 3D hydrogels of three different moduli. (Scale bar, 200 μm.)
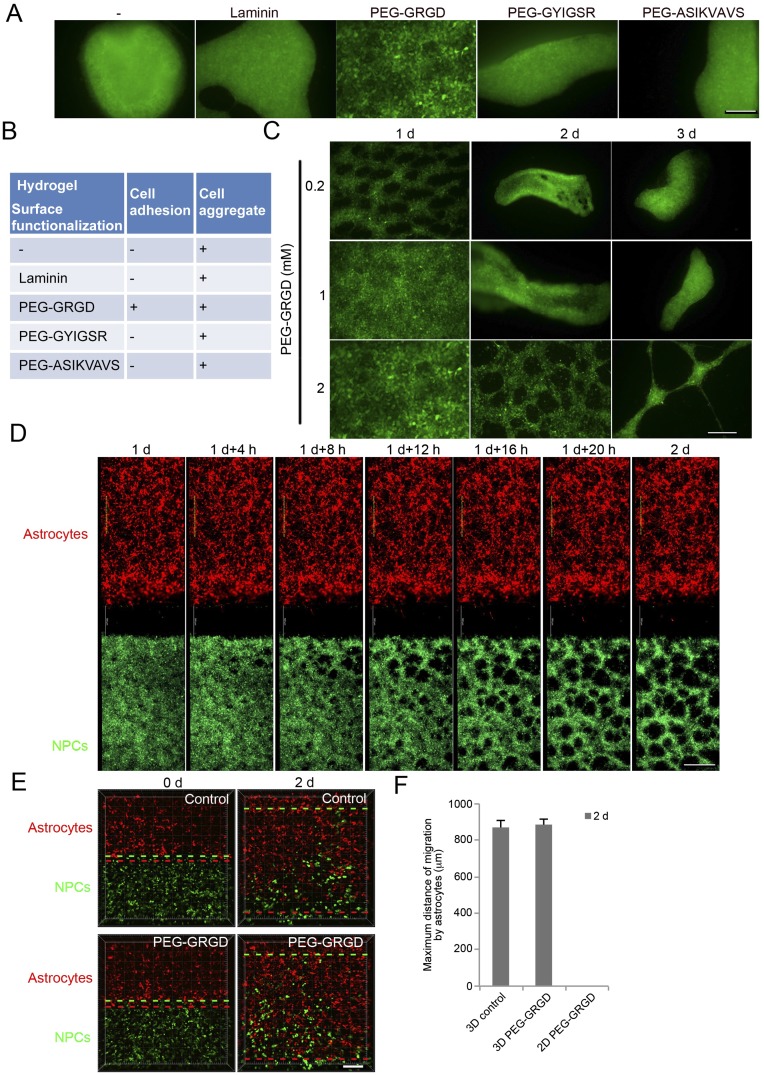
Cell adhesion, spreading, and locomotion on 2D substrates is inversely proportional to substrate elasticity for a wide range of elastic moduli. Native ECM elasticity is tissue-dependent; however, cells are typically rounded, minimally adhesive, growth-arrested and prone to apoptosis when grown on soft 2D matrices (27). For this study, we designed a scaffold to mimic native mechanical and biochemical properties of the developing CNS (25, 26). Not surprisingly, our initial efforts to cultivate NPCs and astrocytes on 2D soft material substrates were unsuccessful. Encapsulating and absorbing laminin into 2D HAMA hydrogels failed to promote attachment and spreading. Instead, NPCs quickly aggregated and detached as spheres (Fig. S7 A and B). It has been reported that cell adhesion peptides and proteins engineered into hydrogels promote neuronal differentiation and outgrowth (24, 28). Therefore, we covalently cross-linked potent integrin-binding adhesion ligands into the hydrogel surface, specifically Arg-Gly-Asp (RGD), Tyr-Ile-Gly-Ser-Arg (YIGSR), and Ile-Lys-Val-Ala-Val (IKVAV), to compliment the innate CD44-binding property of hyaluronic acid. Acryl-PEG3400-GRGD, Acryl-PEG3400-GYIGSR, and Acryl-PEG3400-ASIKVAVS were custom-ordered (21st Century Biochemical and Laysan Bio). We tested cell adhesion on ligand-functionalized 2D HAMA hydrogels 12 h after initial seeding. RGD peptide, but not YIGSR or IKVAV, promoted initial NPC attachment (Fig. S7 A and B). Further, we optimized the RGD concentration-dependent initial adhesion and spreading. Despite initial NPC attachment, NPCs only adhered to “optimized” RGD HAMA surfaces for 2 d before aggregating, and ultimately detached after 3 d (Fig. S7C). Within the initial 2-d period, using time-lapse fluorescence microscopy, we observed no NPC migration toward discretely cocultured astrocytes on 2D RGD HAMA substrates (Fig. S7 D and F). Identical functionalization in 3D RGD HAMA scaffolds had no adverse effect on encapsulated astrocyte-induced NPC migration over the same time period (Fig. S7 E and F).
Fig. S7.
The differentiation and migration of NPCs on 2D soft hydrogel surface. (A) Representative images of WT83 cells after neuronal differentiation on 2D soft hydrogel overnight. Laminin, or 2 mM peptide PEG–GRGD, PEG–GYIGSR, or PEG–ASIKVAVS, was included in HA hydrogels. (Scale bar, 500 μm.) (B) Summary of cell adhesion and cell aggregation of NPCs on 2D soft hydrogel surface. (C) Representative images of WT83 cells after neuronal differentiation on 2D soft hydrogel surface with 0.2 mM, 1 mM, or 2 mM peptide PEG–GRGD. (Scale bar, 500 μm.) (D) Time-lapse images of NPC migration induced by astrocytes for 2 d on 2D soft hydrogel surface. (Scale bar, 500 μm.) (E) Representative images of NPC migration induced by astrocytes in 3D hydrogel with or without 2 mM peptide PEG–GRGD for indicated times. Dotted lines identify the farthest cell of each type; the distance between red and green dotted lines was considered the maximum migration distance. (Scale bar, 200 μm.) (F) Quantification of maximum migration distance induced by astrocytes. Bars represent means (n = 3).
Reduced Neurite Outgrowth and Synapse Number in RTT Neurons in 3D Hydrogel.
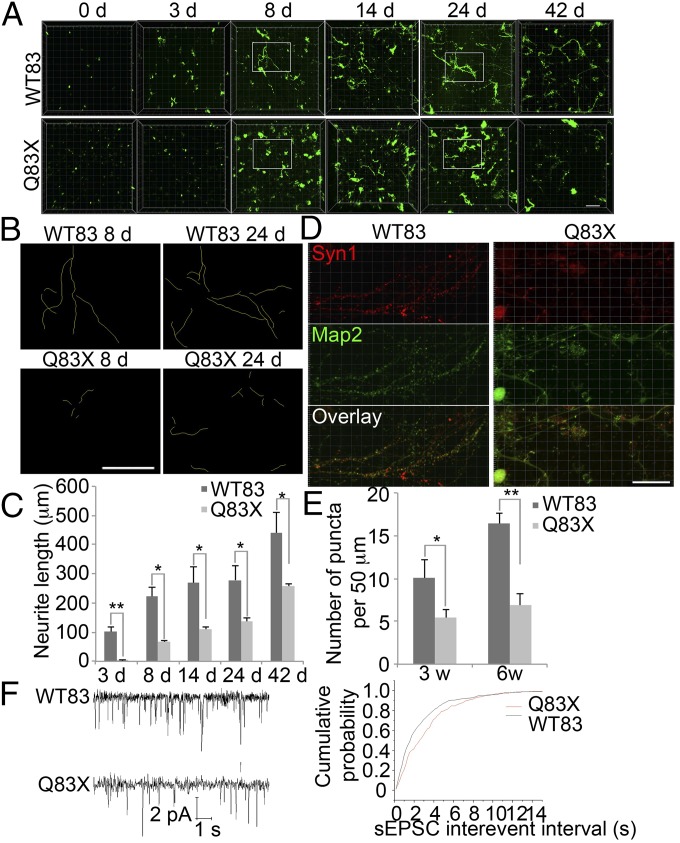
Reduced dendritic arborization has been observed both in RTT patient cortex (29) and in some MeCP2 mutant mice (30), but whether this results from reduced branching during development or from a failure of dendrite maintenance remains unclear. Similarly, disorganization of axons within cortical layers has been observed both in patients and animal models (31). The underlying mechanism behind dendritic arborization could be most easily studied in vitro using human neurons generated from patient iPSCs. We therefore compared neurite outgrowth between MeCP2 Q83X neurons derived from RTT patient iPSCs and control neurons derived from a parent’s iPSCs in our 3D hydrogel system. Neurite outgrowth in Q83X neurons was much slower, producing much shorter neurites than WT83 (Fig. 4 A−C). The difference became apparent at as early as day three and remained significant throughout 42 d of culture (Fig. 4 A−C). To further confirm this difference, we used immunostaining to detect Syn-positive puncta along Map2-positive neurites. Syn puncta were far less dense in Q83X neurons than controls (Fig. 4 D and E), confirming our prior findings in 2D culture (1). To further study the maturation defect, we recorded spontaneous excitatory postsynaptic currents (sEPSCs) as a way of measuring intercellular connectivity and network formation (Fig. 4F). Cumulative probability plots of amplitudes and interevent intervals of spontaneous postsynaptic currents revealed that RTT neurons have a significant decrease in frequency compared with WT neurons (Fig. 4F). Defective neurite outgrowth (Fig. S8 A and C) and a lower density of Syn puncta in Q83X neurons was confirmed using neurons generated from isogenic cell-derived NPCs (Fig. S8 B and D).
Fig. 4.
Defective neurite outgrowth and synapse formation in Q83X neurons in 3D hydrogel. (A) Representative images of WT83 and Q83X cells after neuronal differentiation in 3D hydrogel at different times. (Scale bar, 200 μm.) (B) Neuronal tracing comparing WT83 and Q83X neurons at day 8 and day 24 after differentiation. (C) Quantification of neurite length. Bars represent means of 50 neurons per condition; *P < 0.05, and **P < 0.01; n = 3. (D) Representative images of WT83 and Q83X neurons showing Syn1 puncta on Map2-positive neurites. (Scale bar, 20 μm.) (E) Quantification of Syn1 puncta on Map2 neurites. Bars represent means of 50 neurons per condition; *P < 0.05, and **P < 0.01; n = 3. (F) Spontaneous currents recording of WT83 and Q83X neurons. Cumulative probability plot of interevent intervals (P < 0.05) of sEPSCs from groups of WT83 (black) and Q83X (red) cells shown; n = 5.
Fig. S8.
Isogenic NPCs-derived neurons mimic the neurite outgrowth and synapse formation defects of RTT neurons in 3D. (A) Representative images of isogenic cell-derived neurons after neuronal differentiation in 3D. (Scale bar, 50 μm.) (B) Representative images of isogenic cell-derived neurons showing Syn1 puncta on Map2-positive neurites. (Scale bar, 20 μm.) (C) Quantification of neurite length after neuronal differentiation in 3D. Bars represent means; **P < 0.01; n = 3. (D) Quantification of Syn1 puncta on Map2 neurites. Bars represent means of 50 neurons per condition; **P < 0.01; n = 3.
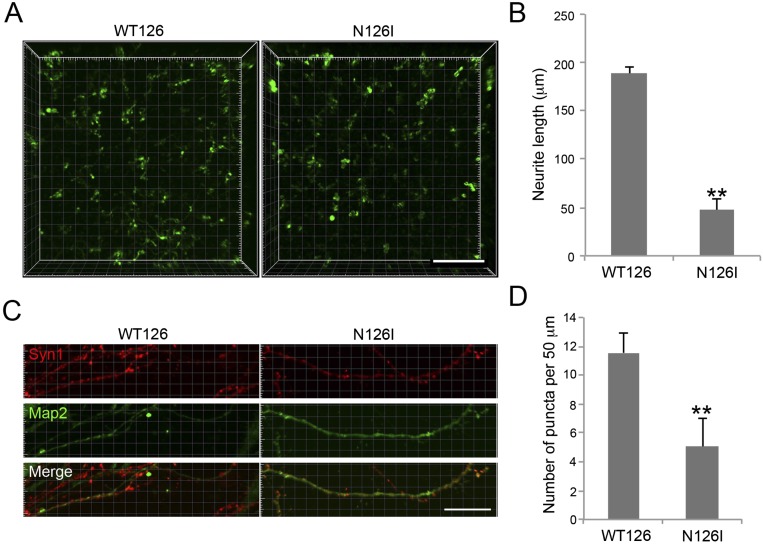
To further confirm the reduced neurite outgrowth and synapse number caused by dysfunction of MeCP2, we used the NPCs derived from another male RTT patient (N126I) and his nonaffected father’s iPSC lines. Neurite outgrowth in N126I neurons was much slower, producing much shorter neurites than WT126 (Fig. S9 A and C). Next, we used immunostaining to detect Syn-positive puncta along Map2-positive neurites. Syn puncta were far less dense in N126I neurons than controls (Fig. S9 B and D). Taken together, our data confirm previous observations of reduced neurite outgrowth and synapse number in RTT neurons.
Fig. S9.
Defective neurite outgrowth and synapse formation in N126I neurons in 3D. (A) Representative images of WT126 and N126I cells after neuronal differentiation in 3D. (Scale bar, 200 μm.) (B) Quantification of neurite length. Bars represent means of 50 neurons per condition; **P < 0.01; n = 3. (C) Representative images of WT126 and N126I neurons showing Syn1 puncta on Map2-positive neurites. (Scale bar, 20 μm.) (D) Quantification of Syn1 puncta on Map2 neurites. Bars represent means of 50 neurons per condition; **P < 0.01; n = 3.
Discussion
Migration of human-derived NPCs has been examined in 3D hydrogels by transplantation into adult rat brains (32), but no method has yet been developed to study 3D migration of human-derived NPCs in vitro. Although NPC migration may be assessed using chemotaxis chambers, such assays may not accurately reflect in vivo differences, as cells can also migrate through aqueous media across membranes rather than specifically along a 3D matrix. The most common in vitro approach for studying 3D migration, coculture with tissue pieces or heterogeneous cell aggregates expressing secreted neurotrophins, has only been applied to tissue explants (33). In this study, we describe a previously unidentified 3D system for manipulating neuronal migration in vitro. This system provides better spatial control and may be more suited to observing single cells than tissue explant cultures.
Rapid generation of neurons in 3D hydrogels could result from several mechanisms. (i) Close matching of the physical properties of the brain [the elastic modus of soft, neurite-supportive hydrogels (100 Pa) is similar to that of brain tissue] (26) may accelerate differentiation. Materials whose mechanical properties closely mimic those of the in vivo ECM of a particular soft tissue have been shown to promote differentiation of progenitor cells into the mature phenotypes inherent to that tissue (34). (ii) The 3D scaffold may allow generation of mechanical force by NPCs in response to their environment (11). (iii) The 3D environment provides a high surface area for growth and migration (35), which can be tuned to support other cell behaviors, such as differentiation or maturation. In vivo-like cell−cell interactions may lead to more realistic gene expression and cellular behavior.
We synthesized cross-linkable HA with the intention to exploit the innate bioactive properties of this high molecular weight glycosaminoglycan naturally abundant in the brain. HA plays a prominent structural role in brain ECM and is upregulated along NPC migratory routes in the developing brain. Many nonneuronal neural cell types express HA receptor CD44, including NPCs and astrocytes (36). Notably, momentary cellular focal adhesions are less stable during HA/CD44 ligation in HA matrices alone compared with α/β integrin-mediated ligation in the added presence of potent ECM ligands such as RGD peptide (37). We encapsulated laminin protein, another key neural ECM component that comprises such ligands, within our HAMA scaffolds to support viability of neural cells. Laminin especially promoted neurite dynamics in differentiating neurons, which cease to express CD44. However, because we did not covalently bind laminin to the matrix, it likely did not significantly contribute to anchorage-dependent NPC locomotion. Native neural ECM varies in character and distribution, with unique molecular structure surrounding a wide variety of neurons. ECM affects both the differentiation efficiency and the derived cell function of developing neural cells (38). Physically entrapping ECM proteins, such as collagen, laminin, or fibronectin, in the synthetic hydrogels has been shown to promote NPC differentiation (39). Although we demonstrated that HAMA hydrogels with laminin only are sufficient to induce NPC differentiation, additional cell adhesion cues might further enhance biomimicry in our 3D system.
In summary, our hydrogel-based assay of neural migration reveals a defect in NPCs derived from an RTT patient’s iPSCs. Further, this hydrogel system facilitates neuronal differentiation and maturation, reducing the time needed to generate functional neurons from over 6 wk to just 3 wk. Using this method to examine migration and differentiation of NPCs derived from patient iPSCs should yield more reliable results than other approaches, as cells move through a soft matrix rather than across a hard surface or through a membrane. Further, it allows exploration of responses to various cell types and biochemical cues without the need for neurosphere culture, which is time-consuming and often inefficient. This system could also be used to screen drug candidates for their ability to restore disease-associated defects in migration or other phenotypes more appropriately studied in 3D systems.
Materials and Methods
For migration, two-layered hydrogels were swollen in medium for 12 h and termed as “0 day.” Live-cell images of the region where the two layers meet were acquired using Olympus confocal microscope. Generation and use of human iPSCs and their derived cells were approved by the University of California, San Diego human research protection program committee meeting under the IRB/ESCRO protocol 141223ZF. All participants gave informed consent to the procedures. All experiments using human materials have been conducted according to the principles expressed in the Declaration of Helsinki. Number of clones from iPSCs used in each experiment in this study is listed in Table S1. Primer sequences for real-time PCR are listed in Table S2. For more materials and methods, please see SI Materials and Methods.
Table S1.
Number of clones from iPSCs used in each experiment in this study
| Subjects | ||||||||||||
| Experiment | WT83 | Q83X | hESC, C1 | hESC–MeCP2 mutation, C1 | Q83X, C1 | rQ83X, C1 | WT126 | N126I | ||||
| C1 | C2 | C1 | C2 | C1 | C2 | C1 | C2 | |||||
| NPC staining | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| NPC FACS | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Neuron differentiation | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Astrocyte differentiation | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Neuron MACS | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Astrocyte FACS | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Neurons/NPCs migration | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Astrocytes/NPCs migration | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Quantitative real-time PCR | 3 | 3 | ||||||||||
| Transmission electron microscopy | 3 | 3 | ||||||||||
| Electrophysiology | 3 | 3 | 3 | 3 | ||||||||
| Western blotting | 3 | 3 | ||||||||||
| Immunostaining | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
The number indicates the times a clone was used for that particular method. C1 and C2 are clone types.
Table S2.
Sequence of primers used in qPCR experiments
| Gene | Primer F | Primer R |
| Nestin | CTGCTACCCTTGAGACACCTG | GGGCTCTGATCTCTGCATCTAC |
| MAP2 | CTCAGCACCGCTAACAGAGG | CATTGGCGCTTCGGACAAG |
| Ctip2 | GGTGCCTGCTATGACAAGG | GGCTCGGACACTTTCCTGAG |
| Syn I | AGTTCTTCGGAATGGGGTGAA | CAAACTGCGGTAGTCTCCGTT |
| VGluT1 | CTGGGGCTACATTGTCACTCA | GCAAAGCCGAAAACTCTGTTG |
| GAPDH | CATGAGAAGTATGACAACAGCCT | AGTCCTTCCACGATACCAAAGT |
SI Materials and Methods
Synthesis of HAMA.
HAMA was prepared following a modified version of reported procedures (11). Hyaluronic acid (91−175 kDa, 1 g, 2 wt%) was suspended in ultrapure water (50 mL), and the resulting mixture was stirred overnight in a cold room at 4 °C until completely solubilized. Dimethylformamide (DMF) (33.5 mL) was then added to obtain 3:2 water/DMF, and the resulting mixture was stirred vigorously in an ice bath at 0 °C. Methacrylic anhydride (0.74 mL, 5 mmol) was added dropwise over 2 h at 0 °C while pH was maintained between 8 and 9 by addition of 0.5 M NaOH, and the reaction was stirred overnight at 4 °C. NaCl (2.4 g, 41 mmol, 0.5 M) was then added to stop the reaction. The polymer was precipitated in cold ethanol (50 mL), and the white suspension was stirred in an ice bath for 30 min. The supernatant was removed by centrifugation at 5,000 × g for 5 min. The resulting white solid was dissolved in ultrapure water (50 mL) and purified by dialysis (Float-A-Lyzer G2 10 mL, 20 kDa; Spectrum Labs) against water for 3 d (water was changed every 8–10 h). Purified HAMA was recovered by freeze-drying (810 mg). The degree of methacrylation (DM) was determined by 1H NMR (600 MHz, D2O, 298 K) by integration of the methacrylate proton signal at 5.7 ppm or 6.2 ppm relative to the acetyl protons signal of HA at 2 ppm; DM = 17 ± 1%. NMR data were acquired at the University of California, San Diego Skaggs School of Pharmacy and Pharmaceutical Sciences NMR facility.
Synthesis of Lithium Acylphosphonate Photoinitiator.
The initiator was synthesized in a two-step process as described in the literature (40). Then 2,4,6-trimethylbenzoyl chloride (3.2 g, 2.9 mL, 17.5 mmol) was added dropwise to dimethyl phenylphosphonite (2.98 g, 2.8 mL, 17.5 mmol) at room temperature (RT) under argon and reacted via a Michaelis–Arbuzov reaction. The reaction mixture was then stirred for 18 h, and a solution of lithium bromide (6.2 g, 70 mmol) in 2-butanone (100 mL) was added. The resulting mixture was then stirred at 50 °C for 10 min. A white precipitate formed, and the resulting suspension was stirred at RT for 4 h and then filtered. The filtrate was washed three times with 2-butanone to remove unreacted lithium bromide and dried under high vacuum to yield lithium acylphosphonate (LAP) as a white powder (4 g, 80%). The photoinitiator was characterized by 1H NMR spectroscopy at 600 MHz in D2O and its purity was confirmed by the presence of peaks at the following chemical shifts (δ) in ppm: 7.71 (dd, 2H, J = 7.9 Hz, 7.5 Hz), 7.56 (t, 1H, J = 7.5 Hz), 7.46 (t, 2H, J = 7.9 Hz), 6.88 (s, 2H), 2.23 (s, 3H), and 2.01 (s, 6H).
Fluorescent Labeling of HAMA.
HAMA (100 mg, DM = 17 ± 1%) was dissolved in ultrapure water (10 mL), and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) (0.2 mg, 1.33 μmol), N-hydroxysulfosuccinimide (NHS) (0.15 mg, 1.33 μmol), and Alexa Fluor 647 hydrazide (0.2 mg, 0.17 µmol) were added. The resulting mixture was stirred overnight in the dark under argon at RT, then transferred to a dialysis membrane (Float-A-Lyzer G2 10 mL, 20 kDa; Spectrum Labs) and dialyzed against water for 3 d (water was changed every 8–10 h). The purified, fluorescent-labeled polymer HAMA-Alexa647 was recovered by freeze-drying (87.5 mg).
Fabrication and Characterization of 3D Hydrogel.
Hydrogels were formulated from HAMA (17 ± 1% methacrylation) with the photoinitiator LAP under UVA irradiation (average intensity of 2.5 mW/cm2, broadband UV centered at 365 nm, UVR-9000; Bayco). Varying HAMA concentrations and addition of various adhesion molecules identified a formulation supporting neuronal differentiation and migration: 1% (wt/vol) HAMA gel and 0.032% (wt/vol) LAP containing laminin (8 μg/mL). Cells were seeded at 15 × 106 /mL in the HAMA solution, which was transferred into 5 mm × 5 mm × 400 µm rubber molds and UV-irradiated for 60 s, 90 s, or 120 s to cross-link. An AFM was used to determine the modulus.
AFM.
Elastic modulus was measured via indentation using an MFP-3D-Bio (Asylum Research) AFM with a 45 μm-diameter polystyrene spherical tip (Novascan) with a nominal spring constant of 0.03 N/m. Hydrogels were mounted on glass slides and kept hydrated in PBS. The spherical probe was indented into the sample with approach and retraction velocities of 2 µm/s to 20 μm/s with a force trigger of 2 nN. Indentation force curves were analyzed using custom code in Igor Pro (Wavemetrics), and elastic moduli were computed using a linearized Hertz model.
SEM.
Hydrogels were placed on a glass coverslip, and excess moisture was removed. Hydrogels were next flash-frozen by immersion in liquid nitrogen, cryofractured using a scalpel blade, and lyophilized overnight. Freeze-dried hydrogel fragments were placed on conductive copper tape on an SEM sample holder, sputter-coated with gold (Cressington 108Auto Sputter Coater), and imaged using a field emission SEM (Agilent 8500 FE-SEM) at 1-kV acceleration.
Fifteen random low magnification micrographs were obtained from each specimen. Micrographs were analyzed for the quantity of synapses. Spine synapses were identified by an electron-dense region associated with vesicles presynaptically and contained a spine apparatus with postsynaptic densities as described previously (41). Approximately 15 electron micrographs (324 µm2) per sample were analyzed in a blinded fashion for total synapse number per area (synapse #/324 µm2).
Transmission Electron Microscopy.
Hydrogels were fixed in 0.15 M cacodylate buffer containing 2.5% (vol/vol) glutaraldehyde, 2% (wt/vol) paraformaldehyde and treated for 1 h with 1% OsO4. Fixed gels were then dehydrated in solutions containing increasing concentrations of ethanol, negatively stained with 2% (wt/vol) uranyl acetate, and embedded in durcupan resin at 60 °C. Thin sections of 70–80 nm were cut with a diamond knife on a Leica ultramicrotome and collected on 300-mesh grids. Sections were stained with 0.3% lead citrate for 1 min and imaged using a transmission electron microscope (FEI Tecnai Spirit G2 BioTWIN) at 80 kV.
NPC Differentiation and Culture.
Fibroblasts from male RTT (Q83X and N126I) patients and their fathers (WT83 and WT126) were reprogrammed and differentiated into NPCs as previously described (1). Two different pairs of control iPSCs and RTT iPSCs were used in our studies to mitigate line-to-line variation. The iPSC colonies were plated on Matrigel-coated (BD Biosciences) plates and maintained in mTESR media (Stem Cell Technologies) for 5 d, after which media was changed to N2 [DMEM/F12 media supplemented with 1× N2 supplement (Life Technologies) and 1 µM dorsomorphin (Tocris)]. After 2 d, colonies were removed and cultured in suspension as embryoid bodies for 2–3 wk using N2 media with dorsomorphin. Embryoid bodies were then gently dissociated with accutase (Gibco), plated on Matrigel-coated dishes, and maintained in DMEM/F12 supplemented with N2, B27, and basic fibroblast growth factor (bFGF) (DMEM/F12 media supplemented with 0.5× N2, 0.5× B27 supplements, 20 ng/mL bFGF, and 1% penicillin/streptomycin). Rosettes emerging after 3 d or 4 d were manually selected, gently dissociated with accutase, and plated in dishes coated with 10 µg/mL poly-ornithine and 5 µg/mL laminin. NPCs were maintained in DMEM/F12 with N2, GEM21, and bFGF. Moreover, we have also generated MeCP2-mutant isogenic cell lines using the clustered regularly-interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9 (Cas9) genome editing (manuscript in preparation). Isogenic stem cell models of RTT were generated by a frame-shift mutation (also found in an RTT patient) in the MECP2 gene in H9 hESCs. Additionally, we rescued a patient cell line with an early stop codon mutation (Q83X), restoring MeCP2 protein levels to normal (rQ83X) using CRISPR/Cas9 genome editing (manuscript in preparation). Number of clones from iPSCs used in each experiment in this study is listed in Table S1.
Astrocyte Differentiation.
Astrocytes were obtained by allowing NPCs to form neurospheres. The bFGF was removed, and Rho-associated protein kinase (ROCK) inhibitor was added for 48 h to induce neuronal differentiation. Spheres were maintained under rotation in neuronal medium for 1 wk followed by 2 wk in astrocytic medium (Lonza) before plating. Spheres were maintained for astrocyte differentiation and later removed to enrich for astrocytes.
Neuronal Differentiation.
In adherent cultures, NPCs were differentiated into neurons by adding 10 μM ROCK inhibitor (Y-27632; Calbiochem) in the absence of bFGF. In 3D hydrogels, NPCs were seeded before polymerization and cultured in NG medium with ROCK inhibitor for 24 h. Medium was changed every other day. To examine whether 3D differentiated neurons were functionally mature, cells differentiated for 3 wk in hydrogels were transferred to glass slides after digestion using hyaluronidase (2,000 units per milliliter) overnight. After 4 d of culture on slides, electrophysiological activity of GFP-positive cells was examined. As hyaluronidase likely does affect neuronal function, 2D differentiated neurons were also treated with hyaluronidase.
Lentivirus Preparation and Infection.
The lentiviral vector was derived from HIV-1 (Invitrogen). The Syn::EGFP and GFAP::tdTomato vectors were obtained by cloning the synapsin-1 promoter (a gift from Dr. G. Thiel, University of the Saarland Medical School, Hamburg, Germany) or the GFAP promoter into the vector (1). CMV::GFP and CMV::tdTomato vectors were obtained by cloning GFP or tdTomato under the CMV promoter into the lentivirus backbone. Lentivirus (Lentiviral Expression System; Invitrogen) was produced according to the manufacturer's instructions. The destination plasmid was cotransfected with the mixture of packaging plasmids into 293FT cells (Invitrogen) using polyethylenimine. The medium was replaced 8 h after transfection with DMEM containing 5% FBS. After 3 d, viral particles in the supernatant were collected, filtered through 0.45-µm filters (Millipore) and concentrated by high-speed centrifuge (90,000 × g, 2 h, 4 °C). Virus solution was stored in aliquots at −80 °C until use.
Western Blotting.
Protein extracts from differentiated cells were resolved on 10% SDS/PAGE gels and transferred to nitrocellulose membranes, which were probed with rabbit polyclonal antibody against GFP (sc-8334, 1:1,000; Santa Cruz Biotechnology) or rabbit polyclonal antibody against Hsp90 (sc-33755, 1:1,000; Santa Cruz Biotechnology). Membranes were subsequently probed with a horseradish peroxidase-conjugated secondary antibody and developed using an ECL detection kit (Amersham Biosciences).
Quantitative Real-Time PCR.
Real-time PCR was performed as previously described (1). Briefly, total RNA was purified from hESCs using an RNeasy Mini kit (74106; Qiagen). Total RNA (1 μg) was reverse-transcribed into cDNA and analyzed by quantitative real-time PCR using FastStart Universal SYBR Green Master (Rox) (04 913 850 001; Roche). Primer sequences are listed in Table S2. The average threshold (33) was determined for each gene and normalized to GAPDH.
Flow Cytometric Analysis.
The human NPCs were fixed, permeabilized, and stained with specific antibodies using standard procedures and analyzed using a Becton Dickinson LSR-II and FACS Diva software as previously described (1). Differentiated astrocytes expressing GFAP::tdTomato were digested using papain and DNase (Worthington Biochemical Corporation) at 37 °C for 30 min, and tdTomato-positive cells were sorted using BD Influx.
Immunostaining and Imaging.
Cells (cultured either on polyornithine- and laminin-coated coverslips or in hydrogels) were fixed with 4% paraformaldehyde in PBS for 15 min at RT. After three washes with PBS, cells were incubated in 0.1% Triton X-100 in PBS for 15 min at RT. After blocking with 1% BSA in PBS for 2 h at RT, cells were incubated with either anti-Nestin (ab51755, 1:500; Abcam), anti-Sox1 (AF3369, R&D, 1:100), anti-Sox2 (2748S, 1:500; Cell Signaling), anti-PAX6 (HPA030775, 1:100; Sigma) anti-Map2 (ab5392, 1:500; Abcam), anti-Tuj1 (T8660, 1:500; Sigma), anti-Syn1 (ab1543p, 1:500; Abcam), anti-Ctip2 (Ab18465, 1:500; Abcam), anti-Tbr1 (Ab31940, 1:250; Abcam), anti-GAD1/GAD67 (MAB5406, 1:500; Abcam), anti-GABA (A0310, 1:500; Sigma), anti-vGlut1 (135.311, 1:500; Synatic System,), anti-TH1 (SC-14007, 1:500; Santa Cruz Biotechnology), anti-GFAP (Z033429-2, 1:500; Dako) or anti-S100β (NBP1-41373, 1:500; Novus Bio) overnight at 4 °C, followed by washing and incubating with the secondary antibody for 45 min in the dark at RT. Coverslips were mounted on glass slides using Vecta shield mounting medium for fluorescence. Stained cells were examined using an Olympus (FV1000) confocal microscope.
Migration Assay.
Two-layered hydrogels, in which NPCs infected with lentivirus encoding a fluorescent reporter were loaded in the bottom layer containing 5% iodixanol and sorted astrocytes or 2D differentiated neurons infected with indicated lentivirus encoding a fluorescent reporter were loaded in the top layer, were crosslinked by UV irradiation (see Fabrication and Characterization of 3D Hydrogel). Hydrogels containing astrocytes were cultured either in NG or AG medium, and those containing neurons were cultured in NG medium. Hydrogels were swollen in medium for 12 h. This day is termed as “0 day.” Live-cell images of the region where the two layers meet were acquired using an Olympus confocal microscope. Z stacks were reconstructed using Imaris software. The width of the region containing both NPCs and astrocytes (or neurons) was measured by marking the farthest cell of each type with a horizontal line and quantifying the distance between them.
Electrophysiology.
For 3D differentiated neurons, we differentiated for 3 wk and transferred them to glass slides by digesting hydrogels using hyaluronidase (2,000 units per milliliter) overnight. After 4 d of culture on slides, electrophysiological activity of cells was examined. Patch clamp recording was performed using an Axopatch 200B amplifier. Whole-cell patch clamp recordings were performed from cells cocultured with mouse astrocytes after 3 wk of differentiation for sEPSC (1). Neurons were identified by Syn::GFP fluorescence. All experiments were performed at RT. Patch pipettes (5–8 MΩ) contained (in millimoles) 150 KCl, 5 NaCl, 1 MgCl2, 2 ethylene glycol tetraacetic acid (EGTA)-Na, and 10 Hepes pH 7.2. The extracellular solution contained (in millimoles) 125 NaCl, 3 KCl, 10 glucose, 26 NaHCO3, 1.2 NaH2PO4, 2 CaCl2, and 1 MgCl2; 1 µM TTX was bath-applied following dilution into the external solution from concentrated stock solutions. All data were acquired with pClamp 10.0 software and analyzed with MatLab v2009b.
Statistical Analysis.
For all viability experiments, a two-tailed Student’s t test was used to evaluate statistical significance, which was noted when *P < 0.05, **P < 0.01, or ***P < 0.005.
Acknowledgments
The authors gratefully acknowledge the NIH New Innovator Awards (DP2OD006499 and DP2OD006495), NIH (5R01EY024134-02), the California Institute for Regenerative Medicine (TR2-01814 and TR4-06747), the International Rett Syndrome Foundation (IRSF Grant 2915 and Grant 2925), a National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Grant (to A.R.M.), and King Abdulaziz City for Science and Technology (through the KACST-University of California, San Diego Center for Excellence in Nanomedicine and Engineering) for funding.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1521255113/-/DCSupplemental.
References
- 1.Marchetto MCN, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143(4):527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deutsch SI, Burket JA, Katz E. Does subtle disturbance of neuronal migration contribute to schizophrenia and other neurodevelopmental disorders? Potential genetic mechanisms with possible treatment implications. Eur Neuropsychopharmacol. 2010;20(5):281–287. doi: 10.1016/j.euroneuro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Khetan S, et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12(5):458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zorlutuna P, et al. Microfabricated biomaterials for engineering 3D tissues. Adv Mater. 2012;24(14):1782–1804. doi: 10.1002/adma.201104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otsuji TG, et al. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Rep. 2014;2(5):734–745. doi: 10.1016/j.stemcr.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6(1):13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeForest CA, Tirrell DA. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat Mater. 2015;14(5):523–531. doi: 10.1038/nmat4219. [DOI] [PubMed] [Google Scholar]
- 8.Choi HK, Won L, Heller A. Dopaminergic neurons grown in three-dimensional reaggregate culture for periods of up to one year. J Neurosci Methods. 1993;46(3):233–244. doi: 10.1016/0165-0270(93)90072-y. [DOI] [PubMed] [Google Scholar]
- 9.Lampe KJ, Antaris AL, Heilshorn SC. Design of three-dimensional engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. 2013;9(3):5590–5599. doi: 10.1016/j.actbio.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30(36):6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Seidlits SK, et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31(14):3930–3940. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 12.Paşca AM, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12(7):671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi SH, et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature. 2014;515(7526):274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MeCP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 15.Chen WG, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302(5646):885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 16.Karpiak JV, Ner Y, Almutairi A. Density gradient multilayer polymerization for creating complex tissue. Adv Mater. 2012;24(11):1466–1470. doi: 10.1002/adma.201103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason HA, Ito S, Corfas G. Extracellular signals that regulate the tangential migration of olfactory bulb neuronal precursors: Inducers, inhibitors, and repellents. J Neurosci. 2001;21(19):7654–7663. doi: 10.1523/JNEUROSCI.21-19-07654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan SH, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6(3):e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scala E, et al. CDKL5/STK9 is mutated in Rett syndrome variant with infantile spasms. J Med Genet. 2005;42(2):103–107. doi: 10.1136/jmg.2004.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, et al. CDKL5, a protein associated with Rett syndrome, regulates neuronal morphogenesis via Rac1 signaling. J Neurosci. 2010;30(38):12777–12786. doi: 10.1523/JNEUROSCI.1102-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neul JL, Zoghbi HY. Rett syndrome: A prototypical neurodevelopmental disorder. Neuroscientist. 2004;10(2):118–128. doi: 10.1177/1073858403260995. [DOI] [PubMed] [Google Scholar]
- 22.Griesi-Oliveira K, et al. Modeling non-syndromic autism and the impact of TRPC6 disruption in human neurons. Mol Psychiatry. 2015;20(11):1350–1365. doi: 10.1038/mp.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariani J, et al. FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell. 2015;162(2):375–390. doi: 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKinnon DD, Kloxin AM, Anseth KS. Synthetic hydrogel platform for three-dimensional culture of embryonic stem cell-derived motor neurons. Biomater Sci-Uk. 2013;1(5):460–469. doi: 10.1039/c3bm00166k. [DOI] [PubMed] [Google Scholar]
- 25.Lu YB, et al. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci USA. 2006;103(47):17759–17764. doi: 10.1073/pnas.0606150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13(18):2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47(4):1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 28.Silva GA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong D, Dunn JK, Antalffy B, Trivedi R. Selective dendritic alterations in the cortex of Rett syndrome. J Neuropathol Exp Neurol. 1995;54(2):195–201. doi: 10.1097/00005072-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Stuss DP, Boyd JD, Levin DB, Delaney KR. MeCP2 mutation results in compartment-specific reductions in dendritic branching and spine density in layer 5 motor cortical neurons of YFP-H mice. PLoS One. 2012;7(3):e31896. doi: 10.1371/journal.pone.0031896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belichenko PV, et al. Widespread changes in dendritic and axonal morphology in MeCP2-mutant mouse models of Rett syndrome: Evidence for disruption of neuronal networks. J Comp Neurol. 2009;514(3):240–258. doi: 10.1002/cne.22009. [DOI] [PubMed] [Google Scholar]
- 32.Englund U, Björklund A, Wictorin K. Migration patterns and phenotypic differentiation of long-term expanded human neural progenitor cells after transplantation into the adult rat brain. Brain Res Dev Brain Res. 2002;134(1-2):123–141. doi: 10.1016/s0165-3806(01)00330-3. [DOI] [PubMed] [Google Scholar]
- 33.Gil V, del Río JA. Analysis of axonal growth and cell migration in 3D hydrogel cultures of embryonic mouse CNS tissue. Nat Protoc. 2012;7(2):268–280. doi: 10.1038/nprot.2011.445. [DOI] [PubMed] [Google Scholar]
- 34.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13(6):645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz KM, Kyburz KA, Anseth KS. Measuring dynamic cell-material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc Natl Acad Sci USA. 2015;112(29):E3757–E3764. doi: 10.1073/pnas.1511304112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindwall C, Olsson M, Osman AM, Kuhn HG, Curtis MA. Selective expression of hyaluronan and receptor for hyaluronan mediated motility (Rhamm) in the adult mouse subventricular zone and rostral migratory stream and in ischemic cortex. Brain Res. 2013;1503:62–77. doi: 10.1016/j.brainres.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y, Kumar S. CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. Mol Cancer Res. 2014;12(10):1416–1429. doi: 10.1158/1541-7786.MCR-13-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma W, et al. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blewitt MJ, Willits RK. The effect of soluble peptide sequences on neurite extension on 2D collagen substrates and within 3D collagen gels. Ann Biomed Eng. 2007;35(12):2159–2167. doi: 10.1007/s10439-007-9389-4. [DOI] [PubMed] [Google Scholar]
- 40.Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials. 2009;30(35):6702–6707. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bamji SX, et al. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40(4):719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]