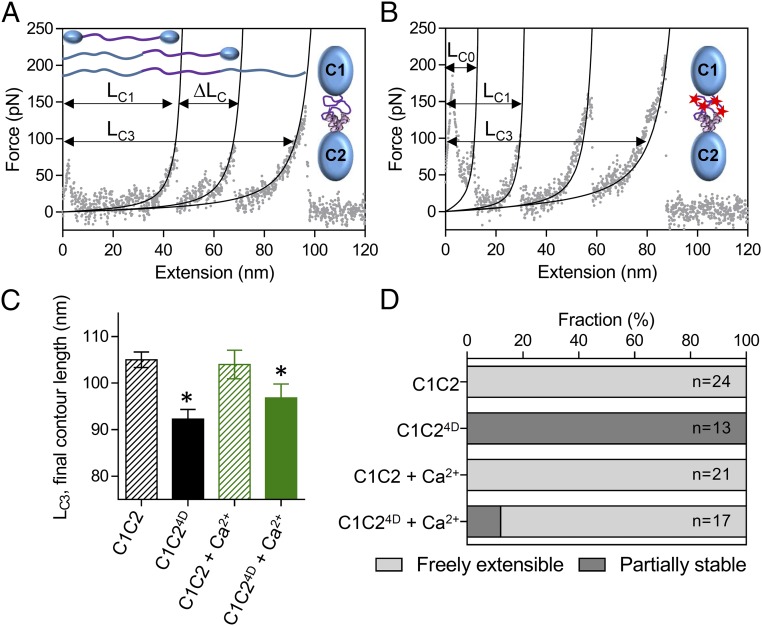
Fig. 3.
Effects of phosphorylation and calcium on the structure of the M-domain. (A and B) AFM force:extension curves for single unphosphorylated wild-type C1C2 (A) and phosphomimetic C1C24D (B) molecules in the absence of calcium. The initial contour length of the freely extensible components (LC1) and final contour length of the fully extended molecules (LC3) are indicated, as is the change in contour length associated with the preceding unfolding event (ΔLC). (C) The final contour lengths of the C1C2 and C1C24D fragments (LC3 in A and B) in the absence and presence 0.1 mM free calcium. *P < 0.01, LC3 vs. C1C2, Student’s t test. (D) The relative stability of the M-domain within the single C1C2 and C1C24D molecules in the absence and presence of 0.1 mM free calcium as determined from the number of molecules showing three (freely extensible M-domain) or four (partially stable M-domain) peaks in the force:extension curves.