The cardiac isoform of myosin-binding protein C (cMyBP-C) is a sarcomeric protein that is believed to be a key regulator of myocardial contractility (review in ref. 1). Although cMyBP-C was thought for many years to be a structural scaffold or template for the cardiac thick filament, there is now considerable evidence for its regulatory function. Previous work has demonstrated reversible phosphorylation of cMyBP-C in vivo in amphibian (2) and mammalian (3) myocardium, as well as phosphorylation-dependent binding of the N terminus of cMyBP-C to cardiac myosin (4) or actin (5). Extraction (6), genetic ablation (7), or phosphorylation (8) of cMyBP-C accelerates contraction. The importance of cMyBP-C to normal cardiac function is highlighted by observations that mutations in the protein account for many cases of heritable hypertrophic cardiomyopathy (9) and by evidence that phosphorylation of the protein is cardioprotective in murine models of transient cardiac ischemia (10).
Although it is known that the N terminus of MyBP-C confers regulatory activity, the molecular mechanisms of regulation via phosphorylation of this region of the protein are not well understood, partly because the secondary structure of the phosphorylatable domain of the protein includes great stretches of random coil and partly because cMyBP-C is able to bind to either myosin or actin. Using complementary cell-free approaches, two reports (11, 12) in PNAS present exquisite insights into the structural effects of phosphorylation on N-terminal regions of cMyBP-C. These results not only suggest specific mechanisms of the protein’s function in vivo but also give rise to new questions that promise to drive research for years to come.
Localization of cMyBP-C in the Cardiac Sarcomere
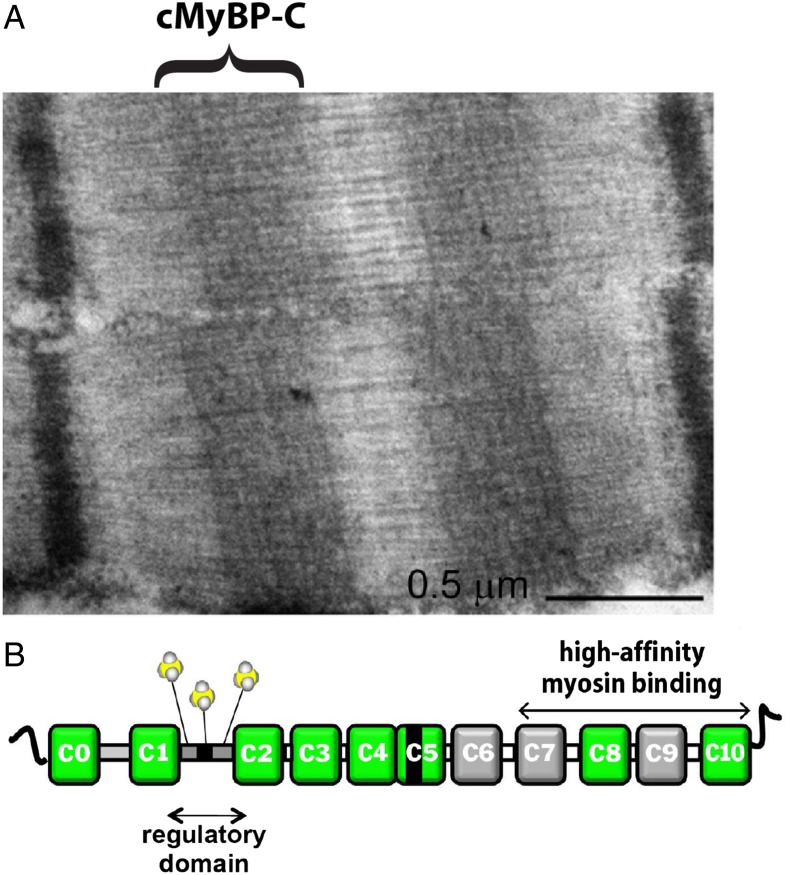
MyBP-C is one of a family of sarcomeric proteins, including X protein and H protein, comprised principally of fibronectin and Ig-like repeats. There are three distinct isoforms of MyBP-C, i.e., fast skeletal, slow skeletal, and cardiac. The localization of cMyBP-C to discrete transverse stripes in each half of the A band (13) (Fig. 1A) is characteristic of the protein in all types of vertebrate striated muscle, and the regularity of its distribution led to the initial suggestion that MyBP-C is a structural protein. This idea has since been refuted in heart muscle by the observation that ablation of murine MYBPC3, the gene encoding cMyBP-C, has no effect on myofibrillar structure, although ablation resulted in structural and functional cardiac phenotypes characteristic of hypertrophic cardiomyopathy (7, 14). A diagram of the structural elements of cMyBP-C is shown in Fig. 1B.
Fig. 1.
(A) Electron micrograph of cardiac sarcomere showing symmetrically localized transverse stripes corresponding to cMyBP-C in the C zone of the thick filaments (13). (B) Diagram of molecular structure of cMyBP-C (1).
Principal Findings and Functional Implications
Beginning in the 1970s, the regulation of cardiac contractility was thought to involve primarily the activation of the thin filaments due to the binding of Ca2+, released from intracellular stores, to troponin C (TnC) in the thin filament regulatory strand. Variations in contractility were explained, at least qualitatively, on the basis of altered Ca2+ binding to TnC as a consequence of adrenergic modulation of Ca2+ release and or Ca2+ binding affinity of cardiac TnC. Although Ca2+ binding to TnC is the essential trigger for activation of the cardiac thin filament, the dramatic depression of inotropic and lusitropic responses in vivo to a β-adrenergic agonist in myocardium expressing nonphosphorylatable cMyBP-C (15, 16) points to a critical regulatory role for cMyBP-C in cardiac contraction.
The present studies by Previs et al. (11) and Colson et al. (12) establish a compelling yet challenging framework for understanding the molecular mechanisms of regulation via phosphorylation of cMyBP-C. Using distinct experimental approaches, the studies have impressive similarities in demonstrating structural changes, i.e., reductions in both length and disorder, in the regulatory M domain near the N terminus of cMyBP-C due to phosphorylation of the three (or four) serines within the domain. Such changes could directly influence the affinity (affinities) of the protein for its binding partners within the sarcomere. For example, phosphorylation has been shown to disrupt the binding of cMyBP-C to myosin in solution (references in ref. 3), which in vivo may release a constraint on myosin and increase its probability of binding to actin, thereby increasing the rate of force development in isolated myocardium or ventricular pressure development.
Both of the present studies also conclude that phosphorylation introduces a bend within the M domain of cMyBP-C and a change in spatial orientation of the N-terminal domains of the molecule. This finding not only accounts for the more compact structure of the domain but may also suggest a mechanism by which the availability of potential binding partners of cMyBP-C may be regulated. Future studies from both groups hold promise for determining whether the pathogenicity of disease-causing mutations in cMyBP-C, such as those in heritable cardiomyopathies, involve disruption of the spatial orientation of the N terminus either in the presence or absence of phosphorylations within the M domain.
Beyond their similarities, each study suggests a unique and novel regulatory mechanism, either or both of which could contribute in important ways to cardiac function or dysfunction. Previs et al. found that elevating Ca2+ to micromolar concentrations in their assays reversed the structural and functional effects due to phosphorylation. This observation is intriguing in at least two ways. First of these is the likelihood that the Ca2+ effect is due to binding to the phosphorylated protein in the absence of a canonical Ca2+ binding site anywhere within its sequence. However, noncanonical coordination of Ca2+ binding has a precedent in muscle, in that scallop muscle contraction is regulated by specific coordination of Ca2+ binding at the confluence of the essential and regulatory light chains and the heavy chain of myosin (17). Although such a precedent does not predict a specific mechanism of Ca2+ binding to phosphorylated cMyBP-C, it does allow for the possibility that such binding occurs.
A second issue that arises from the observation of effects of elevated Ca2+ in the presence of phosphorylated cMyBP-C is the nature of the adaptive advantage of such a mechanism. A plausible answer can be constructed by considering the likely roles played by phosphorylation of cMyBP-C in the responses of the myocardial twitch to a β-adrenergic agonist. Compared with control, the twitch will exhibit a faster rate of force development, increased peak force (positive inotropy), and a faster rate of relaxation (positive lusitropy). However, each of these responses to a β-agonist is sharply blunted in myocardium expressing nonphosphorylatable cMyBP-C (15, 16). The contribution of cMyBP-C to positive inotropy might be explained on the basis of activation of myosin and the thin filament due to a shift in cMyBP-C binding to actin from myosin as a consequence of phosphorylation, described
The structural and functional results reported by Previs et al. and Colson et al. provide an important initial framework for understanding how phosphorylation of cMyBP-C regulates myocardial contraction.
above in relation to the structural changes in the regulatory domain of cMyBP-C reported here. The positive lusitropic responses to a β-agonist presumably involve a number of processes in addition to effects involving cMyBP-C, e.g., accelerated decay of the Ca2+ transient, reduced Ca2+ binding affinity of TnC, and possibly accelerated myosin detachment rates from actin (although there is no evidence to support or refute the latter possibility). If phosphorylation increases the activation of the thin filament by shifting the balance of cMyBP-C binding to actin from myosin, it would be expected to prolong relaxation by promoting cross-bridge binding to actin (negative lusitropy). However, at the micromolar Ca2+ concentrations that are typical of the peak of a β-agonist–stimulated twitch, the present results suggest that Ca2+ binding would induce extension of the M domain even in the presence of phosphorylated residues and presumably disrupt cMyBP-C binding to the thin filament. A consequence of such an event, if it is shown to occur in vivo, would be to reduce the activation state of the thin filament, accelerate cross-bridge detachment, and speed relaxation.
As Previs et al. conclude, their results suggest that activating effects on contraction due to phosphorylation of cMyBP-C are prominent at low-to-intermediate levels of Ca2+ and are reduced or absent at higher levels of Ca2+. This mechanism accounts for the previously unexplained observation that phosphorylation of cMyBP-C accelerates contraction kinetics in permeabilized myocardium at low and intermediate Ca2+ concentrations but not at high (8).
Using all-atom molecular dynamic simulations of the regulatory domains of MyBP-C, Colson et al. observed further structural changes in the M domain due to protein kinase A-mediated phosphorylation of the protein. Their results predict that a sequence of residues that seem to comprise a novel binding site are revealed upon phosphorylation of the protein. Such a site could increase the affinity of the protein for as-yet-unidentified partners, e.g., actin, titin, or even proteins within the thin filament regulatory strand. This is a surprising and intriguing result that begs further study. For example, it appears that, although both the nonphosphorylated and phosphorylated forms of cMyBP-C bind actin, the distortion of the thin filament regulatory strand due to binding differs for the two forms (18). This would be consistent with two distinct binding interfaces depending on phosphorylation state. Another possibility is that exposure of this binding site in vivo results in higher affinity binding of cMyBP-C to the thin filament, which would serve to further increase the effect of cMyBP-C to further activate the thin filament. Alternatively, binding of this site to a partner on the thin filament could conceivably target cMyBP-C or myosin to actin as a mechanism that would in either case increase myosin binding to the thin filament and contribute to the inotropic response to β-adrenergic stimulation.
Conclusions
The structural and functional results reported by Previs et al. (11) and Colson et al. (12) provide an important initial framework for understanding how phosphorylation of cMyBP-C regulates myocardial contraction. Their conclusions that phosphorylation induces a bend in the regulatory domain of cMyBP-C, alters the spatial orientation of N-terminal domains, and exposes a putative novel binding site will stimulate new experiments and contribute to the development of mechanistic models of protein function at multiple levels of spatial organization from molecular to systemic. At the very least, further work promises to define the roles of these processes in cMyBP-C–mediated regulation of myocardial contractility in health and disease.
Acknowledgments
This work was supported by NIH Grant R37 HL82900 (to R.L.M.).
Footnotes
References
- 1.Moss RL, Fitzsimons DP, Ralphe JC. Cardiac MyBP-C regulates the rate and force of contraction in mammalian myocardium. Circ Res. 2015;116(1):183–192. doi: 10.1161/CIRCRESAHA.116.300561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartzell HC. Effects of phosphorylated and unphosphorylated C-protein on cardiac actomyosin ATPase. J Mol Biol. 1985;186(1):185–195. doi: 10.1016/0022-2836(85)90268-2. [DOI] [PubMed] [Google Scholar]
- 3.Weisberg A, Winegrad S. Alteration of myosin cross bridges by phosphorylation of myosin-binding protein C in cardiac muscle. Proc Natl Acad Sci USA. 1996;93(17):8999–9003. doi: 10.1073/pnas.93.17.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautel M, Zuffardi O, Freiburg A, Labeit S. Phosphorylation switches specific for the cardiac isoform of myosin binding protein-C: A modulator of cardiac contraction? EMBO J. 1995;14(9):1952–1960. doi: 10.1002/j.1460-2075.1995.tb07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaffer JF, Kensler RW, Harris SP. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J Biol Chem. 2009;284(18):12318–12327. doi: 10.1074/jbc.M808850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmann PA, Hartzell HC, Moss RL. Alterations in Ca2+ sensitive tension due to partial extraction of C-protein from rat skinned cardiac myocytes and rabbit skeletal muscle fibers. J Gen Physiol. 1991;97(6):1141–1163. doi: 10.1085/jgp.97.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris SP, et al. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res. 2002;90(5):594–601. doi: 10.1161/01.res.0000012222.70819.64. [DOI] [PubMed] [Google Scholar]
- 8.Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res. 2006;99(8):884–890. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- 9.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: From mutation identification to mechanistic paradigms. Cell. 2001;104(4):557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 10.Sadayappan S, et al. Cardiac myosin binding protein C phosphorylation is cardioprotective. Proc Natl Acad Sci USA. 2006;103(45):16918–16923. doi: 10.1073/pnas.0607069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Previs MJ, et al. Phosphorylation and calcium antagonistically tune myosin-binding protein C’s structure and function. Proc Natl Acad Sci USA. 2016;113:3239–3244. doi: 10.1073/pnas.1522236113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colson BA, Thompson AR, Espinoza-Fonseca LM, Thomas DD. Site-directed spectroscopy of cardiac myosin-binding protein C reveals effects of phosphorylation on protein structural dynamics. Proc Natl Acad Sci USA. 2016;113:3233–3238. doi: 10.1073/pnas.1521281113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luther PK, et al. Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J Mol Biol. 2008;384(1):60–72. doi: 10.1016/j.jmb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer BM, et al. Role of cardiac myosin binding protein C in sustaining left ventricular systolic stiffening. Circ Res. 2004;94(9):1249–1255. doi: 10.1161/01.RES.0000126898.95550.31. [DOI] [PubMed] [Google Scholar]
- 15.Tong CW, et al. Phosphoregulation of cardiac inotropy via myosin binding protein-C during increased pacing frequency or beta1-adrenergic stimulation. Circ Heart Fail. 2015;8(3):595–604. doi: 10.1161/CIRCHEARTFAILURE.114.001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosas PC, et al. Phosphorylation of cardiac myosin-binding protein-C is a critical mediator of diastolic function. Circ Heart Fail. 2015;8(3):582–594. doi: 10.1161/CIRCHEARTFAILURE.114.001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houdusse A, Cohen C. Structure of the regulatory domain of scallop myosin at 2 Å resolution: Implications for regulation. Structure. 1996;4(1):21–32. doi: 10.1016/s0969-2126(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 18.Previs MJ, et al. Myosin-binding protein C corrects an intrinsic inhomogeneity in cardiac excitation-contraction coupling. Sci Adv. 2015;1(1):e1400205. doi: 10.1126/sciadv.1400205. [DOI] [PMC free article] [PubMed] [Google Scholar]