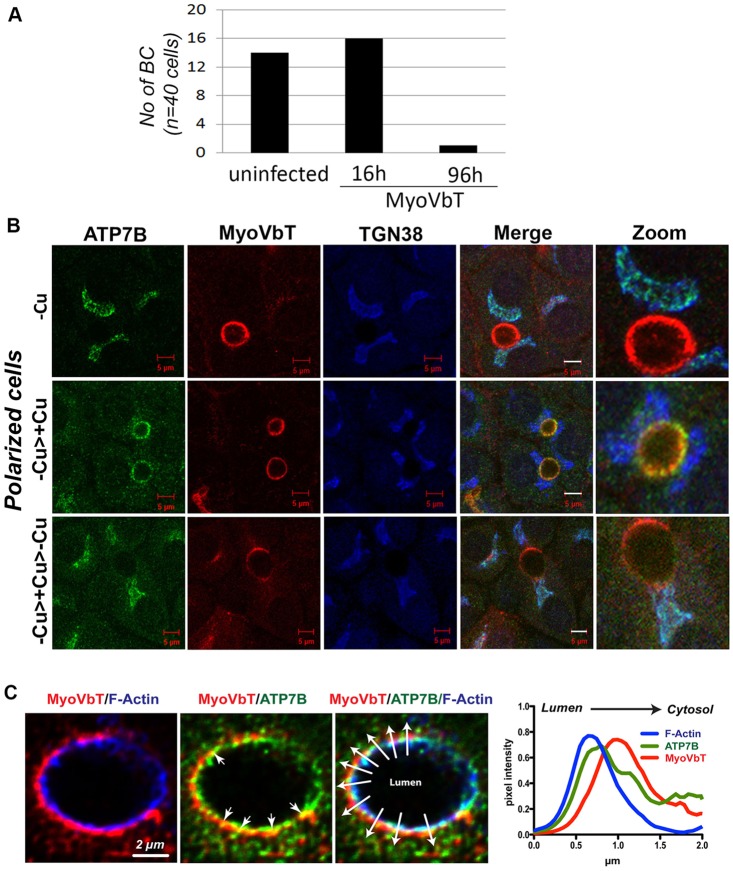
Fig. 2.
Expression of exogenous MyoVbT in polarized WIF-B cells. (A) Histogram showing the number of bile canaliculi (NO of BC) in WIF-B cells infected with Ad-MyoVbT fused to tdTomato for 16 or 96 h (cells were then fixed and bile canaliculi counted). Short-term overexpression (16 h) of MyoVbT in WIF-B cells did not affect the number of bile canaliculi, whereas long-term expression greatly diminished them. (B) WIF-B cells expressing MyoVbT were grown in medium containing low [Cu+] (top row, -Cu), initially grown in medium containing low [Cu+] for 24 h and then transferred to medium containing high [Cu+] for 1.5 h (middle row, -Cu>+Cu), or were initially grown in low [Cu+] for 24 h, transferred to medium containing high [Cu+] for 1.5 h and then transferred to BCS-containing Cu+-free medium for 4 h (bottom row, -Cu>+Cu Cu>-Cu). Cells were triple-stained for ATP7B (green), MyoVbT (red) or TGN38 (blue). MyoVb exhibits an apical ring pattern that is unaltered by Cu+ (second column). ATP7B colocalizes with TGN38 but not with MyoVbT in low [Cu+] (top row). Following Cu+ treatment, ATP7B colocalizes with MyoVbT near the apical ring (middle row). On subsequent Cu+ chelation, ATP7B again overlaps with TGN38 (bottom row). Image detail is shown on the right in all three panels. Scale bars: 5 μm. (C) Spatial organization of ATP7B and MyoVbT near the apical actin ring. MyoVbT surrounds the cytosolic face of the apical F-actin ring labeled by phalloidin (blue, left), whereas ATP7B is distributed between F-actin (blue, right) and MyoVbT (red, middle; arrowheads indicate directionality from lumen to cytosol). Overlapping signals of ATP7B and MyoVbT (yellow) are indicated by arrows. The graph (right) shows the mean normalized pixel intensities for all three markers across the ten lines, with arrowheads shown in the triple-labeled image, plotted to show their spatial distribution relative to the luminal face of the membrane.