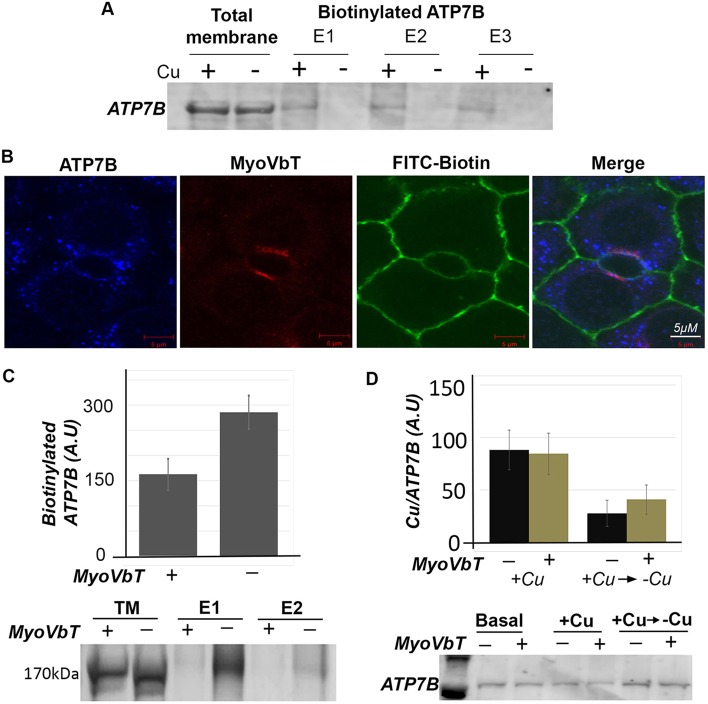
Fig. 5.
Expression of MyoVbT reduces ATP7B at apical surface. (A) Cu+-dependent biotinylation of ATP7B. WIF-B cells grown in high-[Cu+] medium with [10 µM BCS (+)] or in low-[Cu+] medium with [25 µM BCS (−)] were biotinylated on ice, sonicated and cell membranes solubilized with detergent were incubated with streptavidin-coupled beads. Total membrane fractions or eluates from the beads were western blotted for ATP7B. Surface biotinylated ATP7B is detected at high-[Cu+] only. (B) Apical biotinylation in regions of overlap between ATP7B (blue), FITC-biotin (green) and MyoVbT (red) in the apical region of Cu+-treated cells. (C) Histogram shows immunoblot analysis of biotinylated ATP7B comparing cells overexpressing MyoVbT or not in high [Cu+]. Apical biotinylation is reduced in the presence of MyoVbT. The western blots (lower) show ATP7B in the total lysate (lanes 1, 2) or surface biotinylated in the presence (+; lanes 3,5) or absence (−; lanes 4, 6) of MyoVbT. TM denotes total membrane. E1 and E2 signify two serial eluates from the streptavidin agarose beads. (D) MyoVbT slows cellular Cu+ extrusion. Cu+ content of cells expressing MyoVbT (+; yellow bars) or not (−; black bars) was measured by atomic absorption spectroscopy at high [Cu+] or under conditions that had been changed from high to low [Cu+] (addition of BCS). Cu+ uptake appeared normal in MyoVbT-expressing cells (black bars). We observed a modest reduction in the rate of Cu+ extrusion following return to Cu+ chelation in BCS in the presence of myoVbT (yellow bars). Western blotting (bottom) showed similar levels of ATP7B protein under all conditions. Error bars in C and D are the mean±s.e.m.