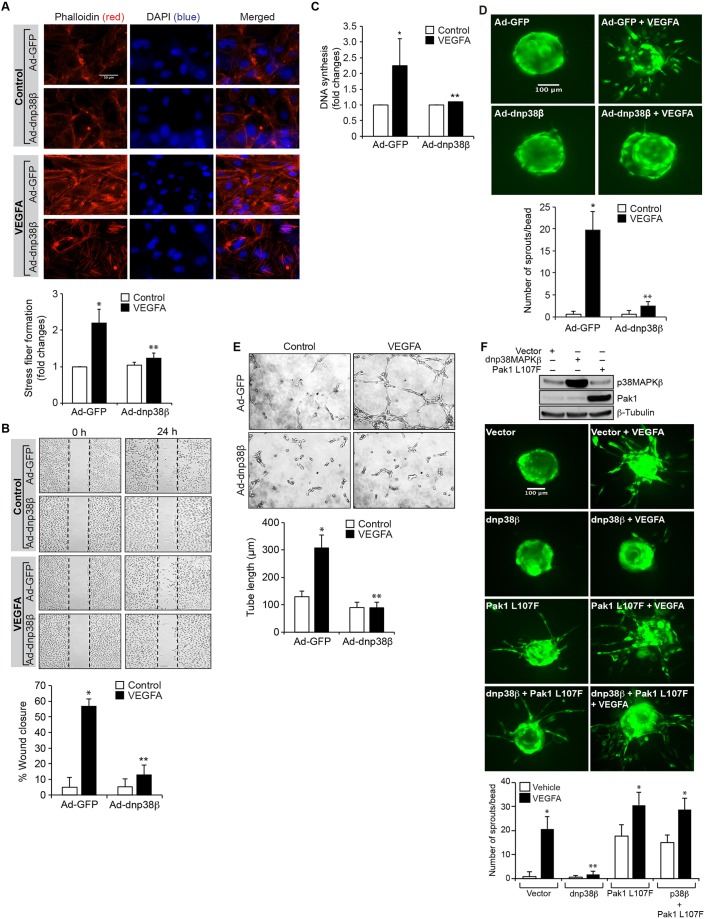
Fig. 5.
p38MAPKβ mediates VEGFA-induced HRMVEC angiogenic events. (A) HRMVECs were transduced with Ad-GFP or Ad-dnp38MAPKβ at 40 MOI, growth-arrested, treated with and without VEGFA (40 ng/ml) for 2 h and stained with phalloidin to assess F-actin stress fiber formation. The changes in stress fiber levels were assessed by measuring the fluorescence intensity, and are presented as the fold changes relative to control. Scale bars: 50 μm. (B–E) All the conditions were the same as in A, except that cells were treated with and without VEGFA (40 ng/ml) and subjected to migration (B), DNA synthesis (C), sprouting (D) or tube formation (E) assays. (F) Upper panel, cells were transfected with vector, dnp38MAPKβ or Pak1 L107F plasmid (1.5 µg/ml). Then, cell extracts were prepared and an equal amount of protein from each condition was analyzed by western blotting for p38MAPKβ or Pak1 levels using their specific antibodies to show their overexpression, and the blot was reprobed for β-tubulin levels for normalization. Lower panel, all the conditions were the same as in the upper panel except that cells were subjected to an VEGFA (40 ng/ml)-induced sprouting assay. The bar graphs represent quantitative analysis of three independent experiments. The values represent mean±s.d. *P<0.01 vs Ad-GFP; **P<0.01 vs Ad-GFP+VEGFA (one-way ANOVA followed by Tukey's post-hoc test).