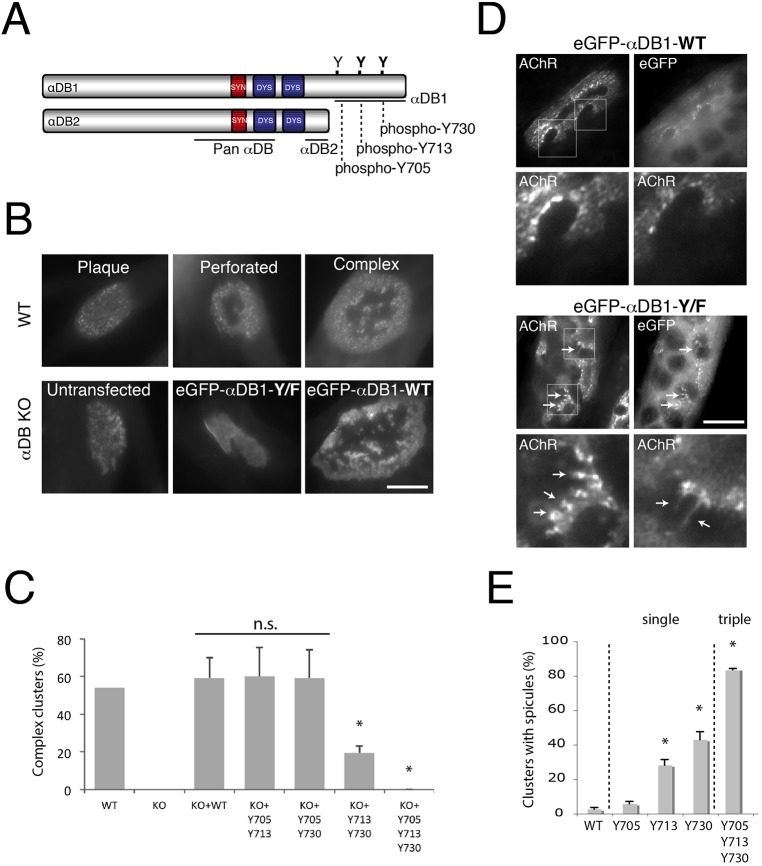
Fig. 1.
Identification of α-dystrobrevin-1 phosphorylation sites involved in postsynaptic maturation. (A) Schematic illustration of α-dystrobrevin-1 (αDB1) and α-dystrobrevin-2 (αDB2) isoforms. The α-dystrobrevin-1 C-terminus bears three tyrosine residues (Y) that can be phosphorylated (Y705, Y713, Y730). Both α-dystrobrevin isoforms contain syntrophin- (red) and dystrophin- (blue) binding domains. (B) Primary myotubes assemble plaques of AChRs that become perforated and mature into complex clusters. Myotubes from α-dystrobrevin-KO (αDB KO) cells form plaques that fail to mature (untransfected). Maturation can be rescued by introduction of wild-type (WT) α-dystrobrevin-1 (eGFP–αDB1-WT), but not mutant α-dystrobrevin-1 (eGFP–αDB1-Y/F) in which Y705, Y713 and Y730 have been mutated to phenylalanine (F) and are unphosphorylatable. (C) Residues Y713 and Y730 are required for the formation of complex clusters. Primary myotubes derived from WT or α-dystrobrevin-KO (KO) myoblasts were transfected with either eGFP–αDB1-WT or mutated α-dystrobrevin-1 constructs and analyzed for their ability to form complex clusters of AChRs. Y705, Y713 and Y730 indicate the mutated residues. Residues Y713 and Y730 are the most crucial for the proper organization of AChR clusters, as shown by the ∼68% decrease in complex clusters quantified [WT: 54%, n=76; αDB-KO: 0%, n=77; αDB-KO+eGFP–αDB1-WT (KO+WT): 59%, n=29; αDB-KO+eGFP–αDB1-Y705-Y713 (KO+Y705 Y713): 60%, n=42; αDB-KO+eGFP–αDB1 Y705-Y730 (KO+Y705 Y730): 59%, n=31; αDB-KO+eGFP–αDB1-Y713-Y730 (KO+Y713 Y730): 19%, n=63; αDB-KO+eGFP–αDB1-Y/F (KO+Y705 Y713 Y730): 0%, n=19]; *P<0.05 (two-tailed Student t-test, mean±s.d.). The percentage of complex clusters was calculated from five independent experiments (each 2–4 wells per construct). n.s., not significant. (D) C2C12 myotubes overexpressing either eGFP–αDB1-WT or the triple mutated (eGFP–αDB1-Y/F) α-dystrobrevin-1 construct were analyzed for aberrations in AChR cluster integrity. Lower images in each set show magnification of the areas boxed in the upper images (AChR channel). Arrows indicate AChR-rich spicules protruding from the edges of the assemblies. (E) Ectopic expression of single mutants in C2C12 myotubes revealed that mutation of residues Y713 and Y730 had the strongest effect on cluster organization. An increasing appearance of spicules was observed in complex clusters bearing Y713, Y730 and Y/F triple mutations (Y705: 5.76%±1.35; Y713: 28.22%±3.26 (P=0.004); Y730: 43.05%±4.56 (P=0.0003); αDB1-Y/F (Y705, Y713, Y730): 83.66%±0.64 (P=0.0001). The percentage of clusters with spicules was calculated from three independent experiments, and at least n=20 clusters were analyzed per each construct (*P<0.05, one-tailed Student t-test, mean±s.d.). Scale bars: 10 μm.