Fig. 3.
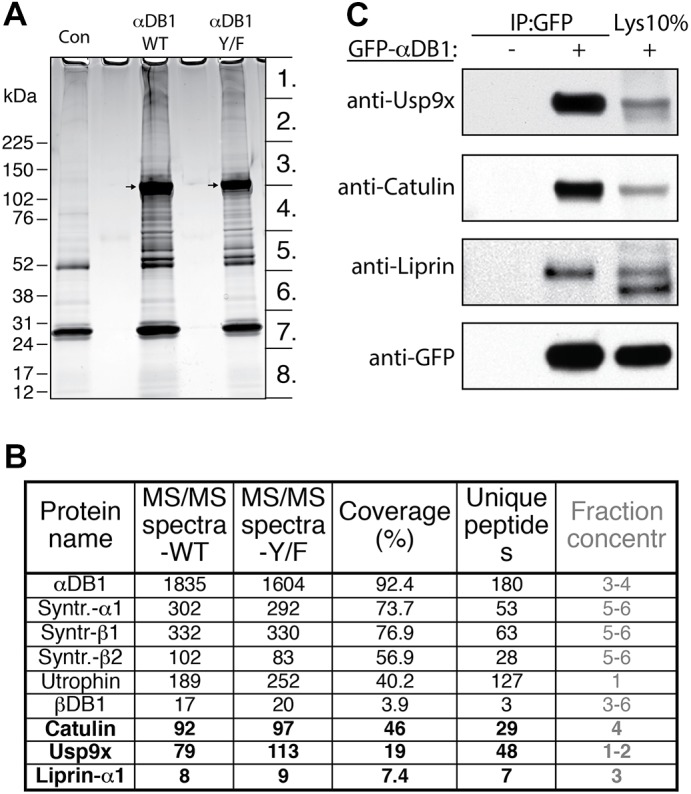
Identification of general α-dystrobrevin-1-binding proteins in myotubes. (A) SDS-PAGE gel of proteins that co-purified with TAP–GFP–αDB1 from C2C12 myotubes. Segments numbered 1–8 show regions analyzed individually with mass spectrometry. Con, control sample obtained from uninfected myotubes; αDB1-WT, sample from myotubes infected with TAP–αDB1-WT virus; αDB1-Y/F sample from myotubes infected with TAP–αDB1-Y/F virus. Arrows indicate αDB1 proteins. (B) Subset of interacting proteins identified with mass spectrometry. Proteins that have not previously been shown to interact with αDB1 are in bold. ‘MS/MS spectra’ for the WT and Y/F constructs represent the total number of peptides identified for each protein; ‘coverage’ represents the percentage of the protein sequence by identified peptides; ‘unique peptides’ represent the number of unique peptides for a given protein. Fraction concentr , fractions of the gel lane (indicated in Fig. 1A) in which protein was detected. (C) Tests of interaction by co-precipitating proteins. Epitope-tagged GFP–αDB1 was transfected into HEK293 cells. Lysates from transfected or untransfected cells (control) were used for immunoprecipitation (IP) with an anti-GFP antibody, and precipitates were analyzed by western blotting (WB) for the indicated proteins. 10% of the lysate (Lys 10%) used for the precipitation experiments was loaded into the gel as a control (right lanes). Syntr., syntrophin.
