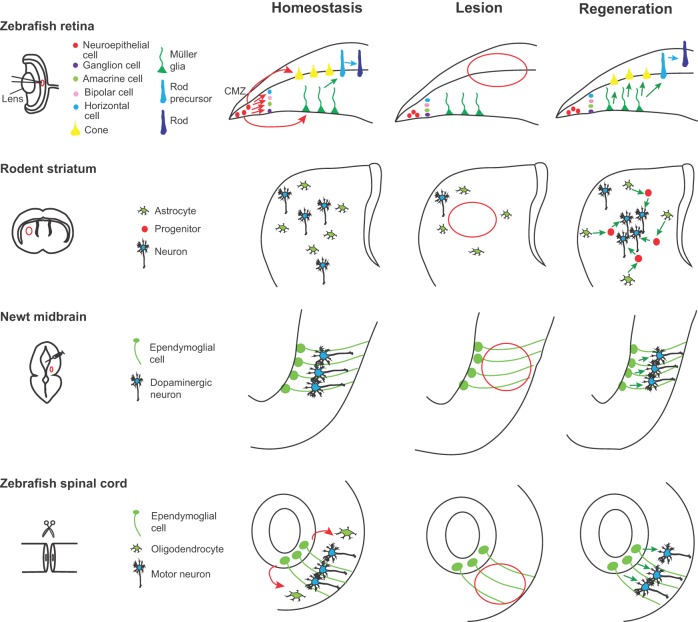
Fig. 3.
Neuronal repair from latent progenitors. In the zebrafish retina, new retinal neurons are generated sequentially from retinal stem cells located in the ciliary marginal zone (CMZ). Under homeostatic conditions, the Müller glia cells (MGs) generate only rod precursors, which give rise to rod photoreceptors (left panel, green and blue arrows). Following lesion (center panel, red outline), MGs re-enter the cell cycle and divide once asymmetrically to generate neurogenic clusters that go on to produce all missing neurons (right panel, green arrows). In the rodent striatum, the astrocytes are not neurogenic (left panel). After a stroke (center panel, red outline), some striatal astrocytes generate neuroblasts that give rise to a limited number of new neurons (right panel, green arrows). In the newt midbrain under homeostatic conditions, ependymoglial cells are quiescent (left panel). A selective neurotoxin administered intraventricularly selectively eliminates midbrain dopaminergic neurons (center panel, red outline), inducing the proliferation of the ependymoglial cells, which generate new dopaminergic neurons (right panel, green arrows). Ependymoglial cells in zebrafish spinal cord are self-renewing and give rise to oligodendrocytes (left panel, red arrows). After a lesion (center panel, red outline), these cells divide, migrate and produce new motor neurons (right panel, green arrows).