Abstract
Study Objectives:
Sleepwalking is a disorder characterized by arousal specifically from slow wave sleep with dissociated brain activity that may be related to lower nociceptive state. Our objectives were to assess the frequency of chronic pain, headache, and migraine in sleepwalkers compared to controls, examine the impact and determinants of pain in sleepwalkers, and report analgesia frequency during injurious parasomnia episodes.
Design:
Cross-sectional case-control study.
Setting:
Data were collected at the Sleep Disorders Center, Montpellier, France.
Participants:
One hundred patients with sleepwalking were assessed for disease characteristics, sleep (polysomnography, sleepiness, and insomnia), pain (chronic pain, multidimensional pain inventory, headache, and migraine), depressive symptoms, and quality of life compared to 100 adult controls. Pain perception was retrospectively assessed during injurious parasomnia episodes.
Measurements and Results:
Raw association data showed that lifetime headache, migraine, and chronic pain at time of study were significantly associated with sleepwalking (also called somnambulism). Compared to controls, sleepwalkers reported more frequent daytime sleepiness, and depressive and insomnia symptoms. After adjustments, sleepwalking was associated with increased risk for headache and migraine only. Compared to pain-free sleepwalkers, sleepwalkers with chronic pain were more likely to be older and to have greater daytime sleepiness, insomnia, and depressive symptoms, with no difference in polysomnography assessment. Of the 47 sleepwalkers with at least one previous violent parasomnia episode, 78.7% perceived no pain during episodes, allowing them to remain asleep despite injury.
Conclusion:
Our results highlight the clinical enigma of pain in sleepwalking patients with complaints of frequent chronic pain, migraine, and headache during wakefulness but who report retrospectively experience of analgesia during severe parasomnia episodes, suggesting a relationship between dissociated brain activity and nociceptive dysregulation.
Citation:
Lopez R, Jaussent I, Dauvilliers Y. Pain in sleepwalking: a clinical enigma. SLEEP 2015;38(11):1693–1698.
Keywords: sleepwalking, parasomnia, pain, headache, analgesia
INTRODUCTION
Disturbed sleep is a major complaint of subjects suffering from chronic pain.1 Pain during sleep increases arousal frequency, thereby disrupting sleep. However, experimental studies also suggest another causal dynamic between pain and sleep disturbances.2 Partial and total sleep deprivation protocols in humans have increased hyperalgesia by decreasing pain perception thresholds.3,4 Selective stage-specific sleep deprivation studies have highlighted the prominent role of both slow wave sleep (SWS) and REM sleep in modulating pain perception.3,5,6
Sleepwalking (also called somnambulism) is a disorder in which only SWS is disrupted. This NREM parasomnia is characterized by inappropriate motor behaviors, usually initiated during arousal from SWS, that induce psychological distress and alter quality of life, leading to fatigue, excessive daytime sleepiness, and objectively impaired vigilance in the morning.7–10 Compared to controls, sleepwalkers had difficulty maintaining stable, consolidated sleep and experienced more arousals and microarousals, specifically from SWS, leading to increased NREM instability, especially during the first sleep cycles.10–12 We hypothesized that alterations in the build-up of slow wave activity may decrease the pain perception threshold and influence pain production in sleepwalking. Except for the frequent association reported with migraine,13 no study to our knowledge has focused on pain experienced during sleepwalking.
Nociception is a complex process involving connectivity between many peripheral and central nervous system structures. Some evidence suggests that sleepwalking is a dissociative state caused by disturbances in local sleep mechanisms due to incomplete arousal from SWS.14–16 Accordingly, dissociated brain activity combined with the persistence of local sleep versus wake state may activate regions involved in nociception and pain perception, such as the thalamocortical network. Our clinical experience suggests that some violent parasomnia behaviors are accompanied by analgesia during episodes, allowing patients to remain asleep despite serious injury. However, no systematic assessment of pain perception during parasomnia episodes has been conducted in sleepwalkers.
The aims of the present study were to (1) compare the frequency of chronic pain, headache, and migraine between sleepwalkers (SW) and controls; (2) examine the impact and determinants of pain in sleepwalkers; and (3) report retrospectively analgesia frequency during injurious parasomnia episodes.
METHODS
Participants
One hundred adults (55 males, 45 females; aged 18–59 years; median age 30 years) were diagnosed with sleepwalking at the Sleep Disorders Clinic, Montpellier, France. Inclusion criteria were (1) a primary complaint and typical clinical history of sleepwalking characterized by behavioral manifestations, misperception of the environment, impaired judgment, and frequent retrograde amnesia (assessed by the patient and bed partner or parent), with no traumatic, neurological, or medication-induced origin according to guidelines; (2) at least one sleepwalking episode annually; and (3) at least one episode in the last 6 months. Exclusion criteria were a positive clinical history of neurological disease (including epilepsy, REM sleep behavior disorder, or parkinsonism) or psychosis. Subjects with at least one concomitant sleep disorder (mainly behaviorally induced insufficient sleep syndrome, narcolepsy, idiopathic hypersomnia, or restless leg syndrome) were excluded by semi-structured clinical interview and video-polysomnography.
A control group of 100 community-dwelling volunteer adults was recruited from local association networks (Montpellier, France). Controls were matched for age (± 1 year) and gender (55 males, 45 females; aged 18 to 58 years; median age 27.5 years). The same exclusion criteria were used for controls, and none reported any current or past symptoms of NREM parasomnia.
All participants gave their informed consent to participate in the study, which was approved by the local institutional review board.
Polysomnography
All sleepwalkers, drug-free, underwent one night of audio-video-polysomnography (PSG) recording in the sleep laboratory, as described previously.9 All PSGs were scored manually for sleep stages, microarousals, periodic limb movements, and respiratory events according to standard criteria.17 Participants with an index of apneas + hypopneas > 10/h or with a periodic limb movements index during sleep associated with micro-arousal > 10/h were excluded. Particular attention was paid to quantify SWS interruptions (microarousals and awakenings in SWS, and hypersynchronous delta wave arousals in SWS per hour of SWS)10 in a subset of patients with PSGs available (n = 57) to be reanalyzed for this issue. No controls underwent PSG recording.
Clinical Assessment
Patients and controls participated in a standardized face-to-face clinical interview to assess demographic features and the presence and characteristics of sleep and pain problems.
Excessive daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS). A total score > 10 indicates excessive daytime sleepiness.18 Insomnia was assessed with the insomnia severity index (ISI), a 7-item self-report scale assessing subjective insomnia symptoms.19 A cutoff score > 14 indicates significant insomnia, and > 21 severe insomnia. Depressive symptoms were assessed with the Beck Depression Inventory (BDI), a 21-item self-assessment tool, with higher scores indicating more severe depressive symptoms (14–19, moderate; > 19 severe).20 Health-related quality of life was assessed with the MOS 36-item Short Form Health Survey (SF-36),21 containing 36 items with 8 scales. Higher SF-36 scores indicate better quality of life.
Pain and Headache Assessment during Wakefulness
Pain was assessed by a clinician and self-report questionnaires. First, patients and controls were asked whether they experienced any chronic pain symptoms. To be chronic, the pain had to be present ≥ 3 months before the time of study. Chronic pain location was identified from a list of 9 preselected body regions: (1) head and face, (2) cervical region, (3) upper limbs, (4) thoracic region, (5) dorsolumbar region, (6) abdominal region, (7) lower limbs, (8) pelvic region, and (9) perineal region. Participants with chronic pain also completed the West Haven-Yale Multidimensional Pain Inventory (WHYMPI),22 containing 5 subscales to assess the impact of pain on the patient's life (pain intensity, pain interference, affective distress, social support, and life control). Scores on each item range from 0 (never or minimum level) to 6 (very frequently or maximum level) and are averaged to obtain subscale scores.
Lifetime headache frequency and headache characteristics were reported by all participants. Recurrent headache disorder manifesting as attacks lasting 4 to 72 h with typical characteristics of the headache (unilateral location, pulsating quality, moderate or severe intensity, aggravation by routine physical activity, and association with nausea and/or photophobia and phonophobia) were diagnosed as migraine, according to guidelines (International Classification of Headache Disorders ICHD-II).23
Pain Perception during Parasomnia Episodes
Violent parasomnia behaviors (physically aggressive or potentially dangerous behaviors for patients and co-sleepers) were systematically investigated in sleepwalkers together with their potential association with injury. Patients with at least one lifetime severe injurious sleepwalking episode associated with pain were asked retrospectively whether they experienced pain either (1) during the parasomnia episode (Question: “Did the pain wake you up from your injurious sleepwalking episode?”) or (2) later during the night or in the morning (Question: “Did you feel the pain only when you woke up later in the night or in the morning?”).
Statistical Analysis
The sample is described using percentages for categorical variables and median and range for quantitative variables, the latter being mostly skewed according to the Shapiro-Wilk test. Sociodemographic and clinical variables between cases and controls were compared using the χ2 test for categorical variables and the Mann-Whitney test for continuous variables.
Associations between pain and headache evaluations (chronic pain, headache, migraine) and sleepwalking were quantified as odds ratios (OR) and their 95% confidence intervals (CI). Sociodemographic and clinical data associated with sleepwalking at P < 0.10 were included in logistic regression models to estimate adjusted OR for the relationship between pain, headache evaluation, and sleepwalking. The same methodology was used to analyze the determinants of pain among sleepwalkers. Significance level was set at P < 0.05. Analyses were performed using SAS statistical software (version 9.3; SAS Inc., Cary, North Carolina, US).
RESULTS
Frequency and Locations of Pain
Sleepwalkers had more frequent daytime sleepiness and depressive and insomnia symptoms compared to controls (Table 1, P < 0.01 for all comparisons). Two patients with sleepwalking met criteria for major depressive disorder, and one for generalized anxiety disorder. No significant differences were found between patients and controls for age, gender, or nocturnal sleep duration. Raw associations showed significant associations between sleepwalking and lifetime headache, migraine, and chronic pain at time of study (Table 2). However after adjusting for ESS, ISI, and BDI scores, sleepwalking was associated with increased risk for headache and migraine only (Model 2: OR = 3.80 95% CI = [1.79–8.07]; OR = 10.04 95% CI = [2.00–50.30], respectively) (Table 2).
Table 1.
Demographic and clinical characteristics of controls and sleepwalkers.
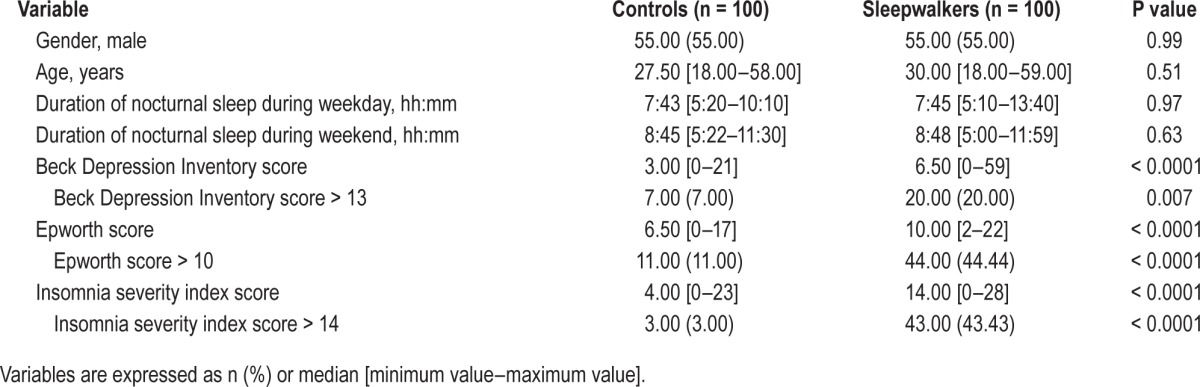
Table 2.
Comparison of chronic pain, headache, and migraine between controls and sleepwalkers.

Chronic pain locations varied greatly, with head, back, and neck being the most frequent for all participants (Figure 1). Wide differences in pain location were found between patients and controls (raw association); however, results remained nonsignificant after adjusting for ESS, ISI, and BDI. No significant associations were found between the 5 scores on the WHYMPI and sleepwalking (Table 3), and results remained unchanged after adjusting for ISI, Epworth, and BDI scores.
Figure 1.
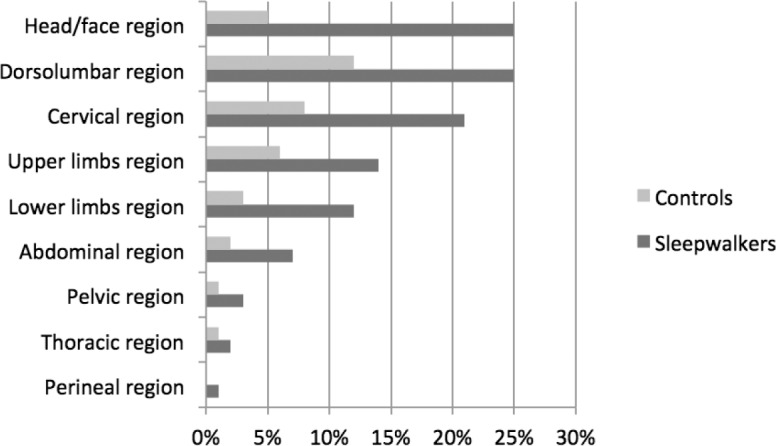
Locations of chronic pain for controls and patients with sleepwalking.
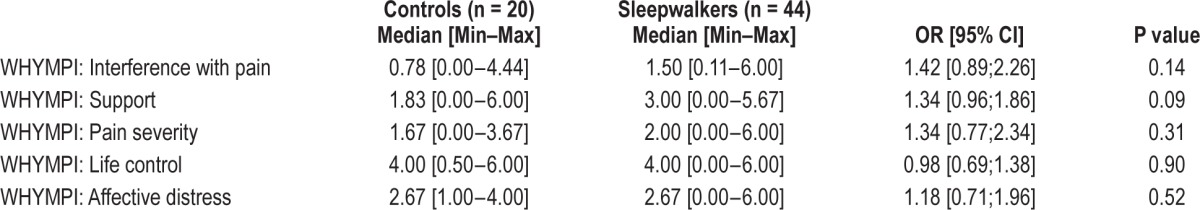
Table 3.
West Haven-Yale Multidimensional Pain Inventory (WHYMPI) scores between controls and sleepwalkers affected with chronic pain - raw association.
Impact of Pain on Sleepwalkers and its Determinants
Of the 100 sleepwalkers, 44 also suffered from chronic pain. Compared to pain-free sleepwalkers, those with chronic pain were more likely to be older (P = 0.02), have higher daytime sleepiness (P < 0.0001), and score higher on the ISI (P = 0.03) and BDI scales (P = 0.002). They were also more likely to be women (P = 0.09). Subsequent analyses of the relationship between chronic pain and both migraine and headache were adjusted for these factors. No significant associations were found between chronic pain and body mass index, frequency of episodes, age at onset of sleepwalking, duration of disease, family history, associated sleep terrors, injurious behavior during episodes, or PSG parameters: total sleep time, sleep efficiency, sleep stage percentages, respiratory events, periodic limb movements, microarousal indexes, and quantification of SWS fragmentation (indexes of microarousals and awakenings in SWS and of hypersynchronous delta wave arousals in SWS per hour of SWS). Concerning the relationship between quality of life scores and chronic pain, sleepwalkers with chronic pain scored low on 5 of the 7 SF-36 subscales (physical functioning P < 0.0001, role-physical P = 0.002, general health P < 0.0001, vitality P = 0.02, and social functioning P = 0.04; bodily pain was excluded from this analysis).
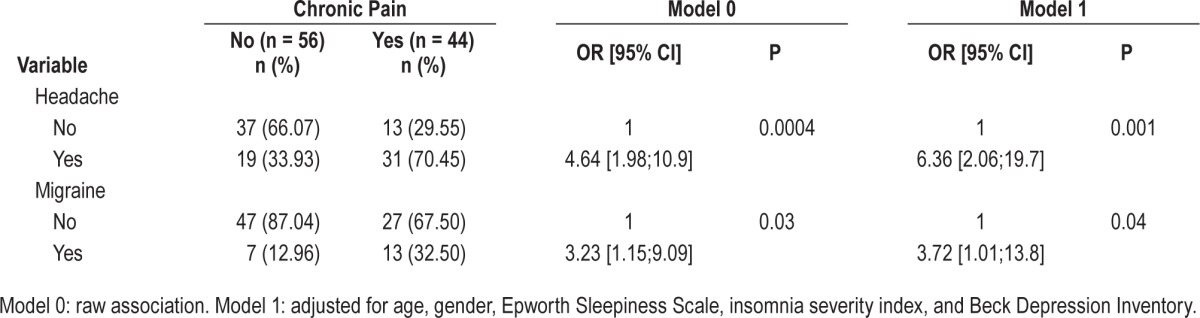
The associations between chronic pain and both headache and migraine are presented in Table 4. Migraine and headache were significantly associated with chronic pain for both models (raw and adjusted: Model 1 OR = 6.36 95% CI = [2.06; 19.7], OR = 3.72 95% CI = [1.01; 13.8], respectively). No demographic or clinical characteristic were associated with migraine in sleepwalkers. Migraine was not associated with higher SWS fragmentation, and it had no impact on sleepwalkers' quality of life. However, patients with both sleepwalking and headache had more violent parasomnia episodes than headache-free sleepwalkers (80% vs 34.78%; P < 0.0001), with no other between-group differences in clinical or PSG parameters.
Table 4.
Relationships between chronic pain and both headache and migraine in sleepwalkers.
Pain Perception during Parasomnia Episodes
Forty-seven patients reported retrospectively having experience of at least one lifetime injurious parasomnia episode associated with pain. Perceived pain during a self-injurious sleepwalking behavior woke the patient immediately in only 10 cases. In contrast, 78.7% of patients (n = 37/47) perceived no pain during episodes, but felt pain later in the night or in the morning after a full awakening. In these cases, the noxious stimuli occurring during an injurious parasomnia did not wake the patient up despite its potential severe intensity. For example, one patient had jumped out a third-floor window during a parasomnia episode, sustaining severe fractures, but perceived the pain only later in the night after being fully awakened. Another patient climbed onto his house roof, fell down, and broke his leg, but did not awaken until morning. The spouse of another patient explained that her husband neither woke up nor complained of pain for several minutes after he had a terrible fall down the stairs during a sleepwalking episode. Accordingly, sleepwalkers frequently present at clinics with painful bruises and contusions after waking up in the morning with no memory of nighttime parasomnia events.
Sleepwalkers with and without reports of analgesia during parasomnia episodes showed no difference on demographic and clinical characteristics, and no associations with migraine, headache, or chronic pain. No between-group differences were found for PSG characteristics, including the quantification of SWS fragmentation. However, due to the small sample size, statistical comparison was impossible.
DISCUSSION
This study demonstrates that almost half the adult sleepwalking patients in our sample experienced either chronic pain or lifetime headache, with migraine in 22%, exceeding the percentages in matched controls. Sleepwalkers had greater daytime sleepiness and depressive and insomnia symptoms than controls, with sleepwalking being associated with increased risk for headache and migraine only, after adjustments. However, almost 80% of sleepwalkers retrospectively reported altered pain perception during severe injurious parasomnia episodes.
Currently, the relationship between sleep and pain remains unclear. Patients with chronic pain frequently have sleep disturbances,24–28 and sleep-deprived conditions reduce the pain threshold.3–6 Sleep and pain interact in complex ways, which may be influenced by several concomitant biological and/or psychological factors. Our results highlight the clinical enigma of pain in sleepwalking patients with complaints of frequent pain during wakefulness but who experience analgesia during parasomnia episodes.
The proportion of sleepwalking patients who suffered from chronic pain was unexpectedly high, at threefold that of controls. The Multidimensional Pain Inventory (WHYMPI) revealed no particular interference with pain, support, pain severity, life control, or affective distress between patients and controls with chronic pain. However chronic pain had a significant impact on sleepwalkers' quality of life. We previously reported reduced quality of life in sleepwalkers compared to age- and gender-matched controls as assessed by scores on the SF-36 scale Bodily Pain scale.9
The locations of chronic pain at time of study varied across patients, with between-group differences, but most subjects suffered from head, back, and/or neck pain. Results showed that the lifetime frequencies of headache and migraine were strongly associated with sleepwalking, with odds ratio as high as 3.8 and 10.04, respectively. These findings confirm previous studies reporting high incidences of sleepwalking and sleep terrors in patients suffering from migraine.13,29–33 However, to our knowledge, no study has reported the frequency of migraine or headache in a large cohort of sleepwalkers. In the present study, no clinical aspects, parasomnia characteristics, or PSG parameters were associated with migraine or headache and sleepwalking, except for more violent parasomnia episodes in patients with headache.
Our current results showed that the association between sleepwalking and chronic pain was modulated by age and daytime sleepiness. Previous studies revealed that chronic pain was frequently reported in insomnia,27 but also in central hypersomnia, which also includes narcolepsy with cataplexy.34 A study also found that sleepy individuals experienced hyperalgesia in response to a painful stimulus compared to non-sleepy individuals with no pain in daily life.35 We previously found more frequent complaints of daytime sleepiness and shorter sleep latencies in the early morning hours in sleepwalkers compared to matched controls.10 However, in this study, we found no between-group (with and without chronic pain) differences in PSG architecture and continuity parameters, insomnia, or depressive symptoms. Against our hypothesis, no association was found between sleepwalkers, slow-wave sleep fragmentation, and chronic pain. Similarly, other studies reported no association between daytime sleepiness and nighttime sleep fragmentation in sleepwalkers.7,10
Despite the widespread prevalence of sleepwalking, its pathophysiology remains poorly understood. The brain is partially awake, resulting in behavioral manifestations, and partially in NREM sleep, resulting in no conscious awareness of actions. A SPECT study of one sleepwalking episode found increased activation in the posterior cingulate cortex and cerebellum, with deactivation in the frontoparietal associative cortices.14 Data from intracerebral EEGs during confusional arousals confirmed both local arousal of the motor and cingulate cortices and increased delta activity in the frontoparietal associative cortices.15,16 As these neuronal networks also play a role in nociceptive regulation, we hypothesize that a dissociated arousal state in these regions may modify the components of sleep-wake behavior, consciousness, and pain perception. Hence, activity inhibition mechanisms within the thalamocortical circuits may block the transfer of sensory information to the cerebral cortex, thus inducing analgesia. Accordingly, 79% of sleepwalkers perceived no pain during violent parasomnia episodes, allowing them to remain “asleep” despite potential serious injury. Studies in healthy volunteers have revealed that nociceptive input processing is attenuated across sleep stages, preventing awakening in response to non-meaningful sensory inputs.36 In sleepwalking, this process may be reinforced by the differing sensitivity to arousing stimuli between the sub-cortical and cortical areas. Loss of the inhibitory function of the frontoparietal cortices together with activation of the motor and cingulate cortices may explain the complex behavioral patterns and the analgesic effect during parasomnia episodes. The abnormal function of arousal circuits in sleepwalkers may also modify cortical excitability, even during wakefulness. Using transcranial magnetic stimulation, a previous study reported altered excitability of the motor cortex during wakefulness in sleepwalkers, suggesting impaired GABA-A neurotransmission.37 GABA-A is a good candidate to explain the potential impaired efficiency of inhibitory subcortical circuits in sleepwalking, as it constitutes an important inhibitory neurotransmitter, and GABAergic neurons play a key role in NREM sleep processing38,39 and nociception regulation.40,41 The large difference in GABA-A receptor distribution in the brain with lower density in subcortical structures (including the thalamus) compared to the cortex may also explain the variable response to noxious stimulation according to dissociated brain activity in sleepwalking.42–44 All these findings strengthen the argument for potential underlying mechanisms associated with frequent observations of analgesia during severe injurious parasomnias which may lead to serious medical and legal complications. If confirmed by either further prospective investigations or objective measurements of pain sensitivity during sleep and parasomnia episodes, our results contrasted with the frequent argument that subjects would have been awakened due to pain during violent behavior which possibly occurred while sleepwalking with major consequences for the complex field of forensics.
Several limitations in our study need to be addressed. First, the etiology and mechanisms underlying chronic pain were not individualized in sleepwalkers. Second, because sleepwalking episodes are frequently associated with lack of conscious awareness and memory of the event, we cannot formally exclude that the absence of perceived pain during parasomnia episodes is due to a recall bias.45 The retrospective assessment of perceived pain during parasomnia episodes over a lifetime span may also introduce a recall bias. Third, PSG assessment for SWS fragmentation was not available for all patients, which may have decreased the statistical power of the analysis. Fourth, our selection criteria excluded patients with sleepwalking comorbid with significant sleep disordered breathing or periodic limb movements index during sleep, restless legs syndrome, hypnotic medications (i.e., benzodiazepine receptor agonists) that preclude generalization of our findings to all sleepwalkers. Finally, we were unable to measure pain sensitivity in the present study.
Our results show increased frequency of chronic pain, migraine, and headache together with altered pain perception during parasomnia episodes in patients with sleepwalking, suggesting a relationship between dissociated brain activity and the co-occurrence of local awakenings, local sleep, and nociceptive dysregulation. We may conclude that greater attention should be paid to assessing pain in sleepwalkers.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Dauvilliers has received funds for speaking and board engagements with UCB Pharma, Jazz, and Bioprojet. The other authors have indicated no financial conflicts of interest.
Footnotes
A commentary on this article appears in this issue on page 1667.
REFERENCES
- 1.Moldofsky H. Sleep and pain. Sleep Med Rev. 2001;5:385–96. doi: 10.1053/smrv.2001.0179. [DOI] [PubMed] [Google Scholar]
- 2.Lautenbacher S, Kundermann B, Krieg J-C. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Onen SH, Alloui A, Gross A, et al. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 4.Lavigne GJ. Effect of sleep restriction on pain perception: towards greater attention! Pain. 2010;148:6–7. doi: 10.1016/j.pain.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–92. [PubMed] [Google Scholar]
- 7.Desautels A, Zadra A, Labelle MA, et al. Daytime somnolence in adult sleepwalkers. Sleep Med. 2013;14:1187–91. doi: 10.1016/j.sleep.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Labelle MA, Desautels A, Montplaisir J, Zadra A. Psychopathologic correlates of adult sleepwalking. Sleep Med. 2013;14:1348–55. doi: 10.1016/j.sleep.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Lopez R, Jaussent I, Scholz S, et al. Functional impairment in adult sleepwalkers: a case-control study. Sleep. 2013;36:345–51. doi: 10.5665/sleep.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez R, Jaussent I, Dauvilliers Y. Objective daytime sleepiness in patients with somnambulism or sleep terrors. Neurology. 2014;83:2070–6. doi: 10.1212/WNL.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 11.Gaudreau H, Joncas S, Zadra A, Montplaisir J. Dynamics of slow-wave activity during the NREM sleep of sleepwalkers and control subjects. Sleep. 2000;23:755–60. [PubMed] [Google Scholar]
- 12.Guilleminault C, Poyares D, Aftab FA, et al. Sleep and wakefulness in somnambulism: a spectral analysis study. J Psychosom Res. 2001;51:411–6. doi: 10.1016/s0022-3999(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 13.Mick BA. Headaches and sleepwalking. JAMA. 1974;229:393. doi: 10.1001/jama.229.4.393b. [DOI] [PubMed] [Google Scholar]
- 14.Bassetti C, Vella S, Donati F, et al. SPECT during sleepwalking. Lancet. 2000;356:484–5. doi: 10.1016/S0140-6736(00)02561-7. [DOI] [PubMed] [Google Scholar]
- 15.Terzaghi M, Sartori I, Tassi L, et al. Evidence of dissociated arousal states during NREM parasomnia from an intracerebral neurophysiological study. Sleep. 2009;32:409–12. doi: 10.1093/sleep/32.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terzaghi M, Sartori I, Tassi L, et al. Dissociated local arousal states underlying essential clinical features of non-rapid eye movement arousal parasomnia: an intracerebral stereo-electroencephalographic study. J Sleep Res. 2012;21:502–6. doi: 10.1111/j.1365-2869.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 22.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23:345–56. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 23.Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 2nd ed. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 24.Affleck G, Urrows S, Tennen H, et al. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–8. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson JH, Ancoli-Israel S, Slate MA, et al. Subjective sleep disturbance in chronic back pain. Clin J Pain. 1988;4:225–32. [Google Scholar]
- 26.Mahowald ML, Mahowald MW. Nighttime sleep and daytime functioning (sleepiness and fatigue) in well-defined chronic rheumatic diseases. Sleep Med. 2000;1:179–93. doi: 10.1016/s1389-9457(00)00029-0. [DOI] [PubMed] [Google Scholar]
- 27.Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14:311–4. doi: 10.1097/00002508-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Raymond I, Nielsen TA, Lavigne G, et al. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;92:381–8. doi: 10.1016/S0304-3959(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 29.Barabas G, Ferrari M, Matthews WS. Childhood migraine and somnambulism. Neurology. 1983;33:948. doi: 10.1212/wnl.33.7.948. [DOI] [PubMed] [Google Scholar]
- 30.Dexter JD. The relationship between disorders of arousal from sleep and migraine. Headache. 1986;26:322. [Google Scholar]
- 31.Casez O, Dananchet Y, Besson G. Migraine and somnambulism. Neurology. 2005;65:1334–5. doi: 10.1212/01.wnl.0000180937.20774.20. [DOI] [PubMed] [Google Scholar]
- 32.Isik U, Ersu RH, Ay P, et al. Prevalence of headache and its association with sleep disorders in children. Pediatr Neurol. 2007;36:146–51. doi: 10.1016/j.pediatrneurol.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Fialho LMN, Pinho RS, Lin J, et al. Sleep terrors antecedent is common in adolescents with migraine. Arq Neuropsiquiatr. 2013;71:83–6. doi: 10.1590/s0004-282x2013005000006. [DOI] [PubMed] [Google Scholar]
- 34.Dauvilliers Y, Bayard S, Shneerson JM, et al. High pain frequency in narcolepsy with cataplexy. Sleep Med. 2011;12:572–7. doi: 10.1016/j.sleep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Chhangani BS, Roehrs TA, Harris EJ, et al. Pain sensitivity in sleepy pain-free normals. Sleep. 2009;32:1011–7. [PMC free article] [PubMed] [Google Scholar]
- 36.Lavigne G, Zucconi M, Castronovo C, Manzini C, Marchettini P, Smirne S. Sleep arousal response to experimental thermal stimulation during sleep in human subjects free of pain and sleep problems. Pain. 2000;84:283–90. doi: 10.1016/s0304-3959(99)00213-4. [DOI] [PubMed] [Google Scholar]
- 37.Oliviero A, Della Marca G, Tonali PA, et al. Functional involvement of cerebral cortex in adult sleepwalking. J Neurol. 2007;254:1066–72. doi: 10.1007/s00415-006-0489-0. [DOI] [PubMed] [Google Scholar]
- 38.Gallopin T, Fort P, Eggermann E, et al. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–5. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 39.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–85. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 40.Jasmin L, Rabkin SD, Granato A, et al. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–20. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- 41.Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- 42.Braestrup C, Albrechtsen R, Squires RF. High densities of benzodiazepine receptors in human cortical areas. Nature. 1977;269:702–4. doi: 10.1038/269702a0. [DOI] [PubMed] [Google Scholar]
- 43.Akbarian S, Huntsman MM, Kim JJ, et al. GABA-A receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex. 1995;5:550–60. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- 44.Juszczak GR. Desensitization of GABAergic receptors as a mechanism of zolpidem-induced somnambulism. Med Hypotheses. 2011;77:230–3. doi: 10.1016/j.mehy.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 45.Pressman MR. Sleepwalking, amnesia, comorbid conditions and triggers: effects of recall and other methodological biases. Sleep. 2013;36:1757–8. doi: 10.5665/sleep.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]


