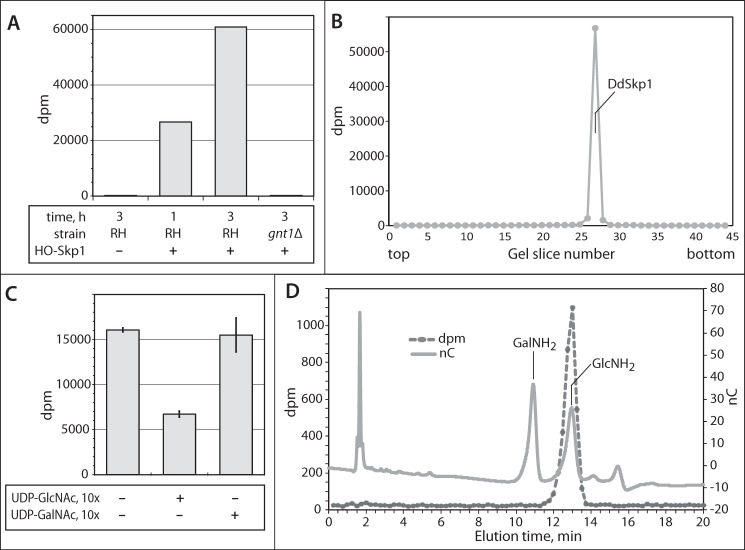
FIGURE 5.
TgGnt1 is a Skp1 GlcNAcT. A, GlcNAcT activity in S100 cytosolic parasite was assayed based on transfer of 3H from 0.5 μm UDP-[3H]GlcNAc to exogenous HO-DdSkp1 for 1–3 h as described under “Experimental Procedures.” Reactions were loaded onto and separated on an SDS-polyacrylamide gel, and the Coomassie Blue-stained DdSkp1 bands were excised and subjected to liquid scintillation spectroscopy. The reaction time, presence of HO-Skp1, and source of the extract (RHΔΔ or RH, or RHgnt1Δ or gnt1Δ) were varied as indicated. B, entire lane from a parallel 3-h reaction (RH, +HO-Skp1) from A was analyzed for incorporation of 3H. Incorporation was only detected at the migration position of DdSkp1. C, donor substrate specificity of the GlcNAcT activity was examined by including a 9-fold excess of unlabeled UDP-GlcNAc or UDP-GalNAc to reactions containing 10 μm UDP-[3H]GlcNAc. Incorporation was measured as in A. Error bars show standard deviations of the mean of two replicates from each of two independent reactions. D, analysis of incorporated 3H. The reacted Skp1 band was excised from a PVDF membrane electroblot of the SDS-polyacrylamide gel, subjected to acid hydrolysis in 6 n HCl, and analyzed by high pH anion exchange chromatography. The hydrolysate was supplemented with GlcNH2 and GalNH2 and chromatographed on a Dionex PA-1 column. Elution of the sugar standards was monitored by a pulsed amperometric detector (nC), and fractions were collected to monitor the elution of 3H by scintillation counting (dpm).