FIGURE 2.
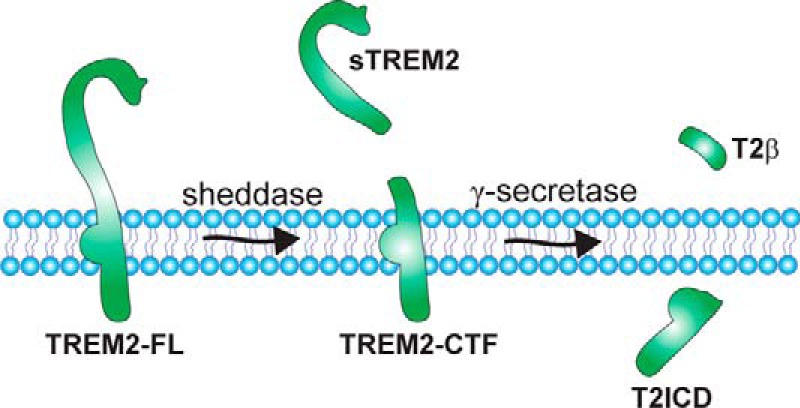
Sequential proteolytic processing of TREM2. The full-length TREM2 receptor (TREM2-FL) can be cleaved by a shedding protease of the ADAM family (sheddase), resulting in the secretion of a soluble ectodomain (sTREM2) and generation of a membrane-bound C-terminal fragment (TREM2-CTF). This C-terminal fragment represents a substrate for γ-secretase-dependent intramembrane proteolysis. The putative cleavage products resulting from γ-secretase-dependent cleavage are indicated as T2β (TREM2-A β-like peptides) and T2ICD (TREM2 intracellular domain). Potential implications of the proteolytic processing for TREM2 function are discussed in the text.
