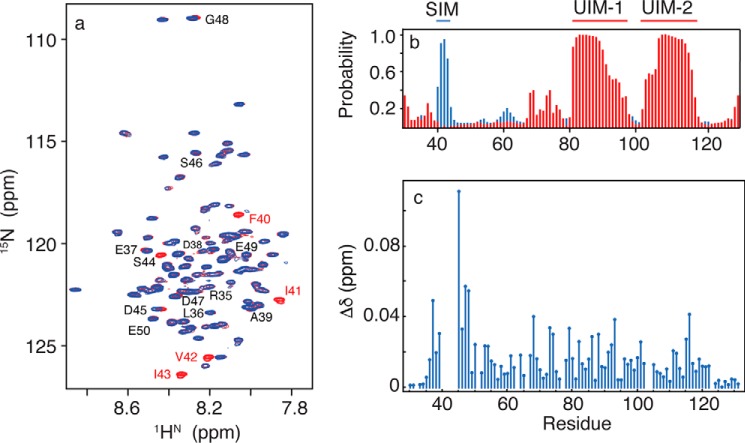
FIGURE 1.
a, two-dimensional 1H-15N HSQC NMR spectra for free RAP80-(33–131) (red) and bound to SUMO-2 (blue). Residues 40–44 are broadened beyond detection upon SUMO-2 binding. b, secondary structure for free RAP80-(33–131) derived from quantitative chemical shift analysis, with α-helix shown in red, and β-strand shown in blue. c, chemical shift perturbations for RAP80-(33–131) bound to an 11-fold excess of SUMO-2.