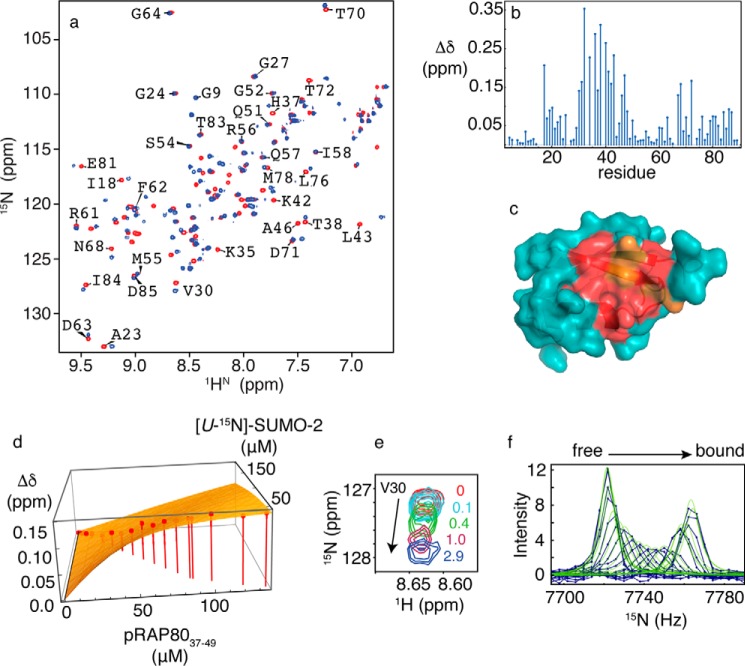
FIGURE 5.
a, two-dimensional 1H-15N HSQC NMR spectra for free SUMO-2 (red) and bound to doubly phosphorylated RAP80-(37–49) (blue). b, chemical shift perturbations for SUMO-2 bound to a 3-fold excess of pRAP80-(37–49). c, residues experiencing chemical shift changes greater than 1 S.D. from the mean (red) and those broadened beyond detection (orange) are mapped on the surface of SUMO-2 (PDB code 1WM2). d, fits of chemical shift changes to 1:1 binding isotherms for the SUMO-2 interaction with pRAP80-(37–49). Chemical shift changes for Val30 are indicated on the vertical axis, and concentrations for SUMO-2 and unlabeled pRAP80-(37–49) are indicated on the horizontal axes. Experimentally determined chemical shift changes are shown as points, and the best fit to a 1:1 binding isotherm is shown as a surface. e, expanded region from two-dimensional 1H-15N HSQC NMR spectra taken during titration of SUMO-2 with pRAP80-(37–49), with the ligand/protein ratios indicated. f, lineshape analysis for Val30 15N chemical shift changes taken from two-dimensional 1H-15N HSQC NMR spectra taken during titration of SUMO-2 with pRAP80-(37–49), experimental data are shown as blue dots connected by lines, and the best fits are shown as green lines.