FIGURE 6.
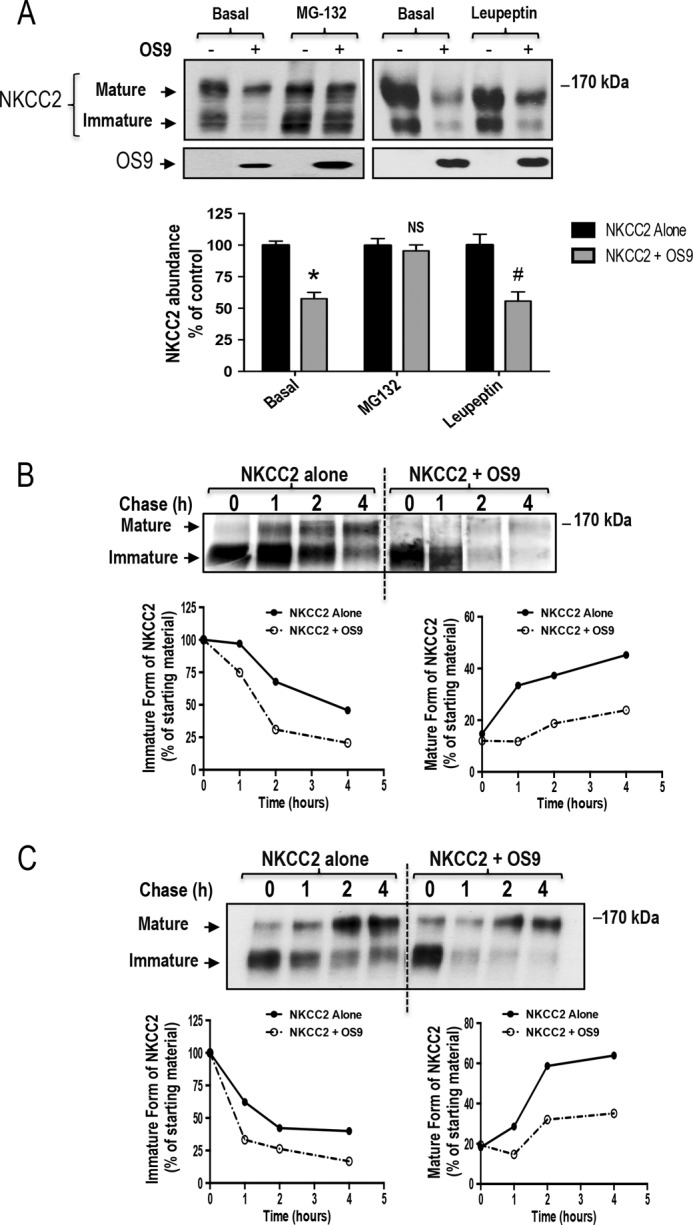
OS9 promotes NKCC2 degradation. A, OS9 decreases NKCC2 expression in a proteasome-dependent manner. OKP cells transiently transfected with Myc-NKCC2 alone or with OS9-V5 were treated with (+) or without (−) 10 μm MG132 or 100 μm leupeptin for 12–16 h prior to cell lysis. The cell lysates were subjected to SDS-PAGE and immunoblotted with anti-Myc and anti-V5 antibodies. Bottom, densitometric analysis of NKCC2 bands from untreated (−) cells and cells treated (+) with MG132 or leupeptin. Data are expressed as percentage of control ±S.E. *, p < 0.0001 versus control (n = 8); NS, not significant versus control (n = 9); #, p < 0.02 versus control (n = 3). B, pulse-chase experiments performed in OKP cells transfected with the indicated plasmids. Cells were labeled with [35S]methionine/cysteine and harvested at the indicated chase times for Myc-NKCC2 immunoprecipitation. Signals were detected by autoradiography. Lower left panel, quantitative analysis of immature NKCC2. The density of the immature form of NKCC2 protein was normalized to the density at time 0 (100%). Lower right panel, quantitative analysis of NKCC2 maturation. In these experiments, we compared the amount of the newly synthesized NKCC2 (immature form at time 0) and the conversion to the complex glycosylated form of the co-transporter (mature form) during the chase period. C, cycloheximide-chase analysis of NKCC2 in the presence or absence of OS9-V5. 14–16 h post-transfection, OKP cells transiently expressing WT NKCC2 alone or in combination with OS9 were chased for the indicated times after addition of cycloheximide. Total cell lysates were separated by SDS-PAGE and probed by anti-Myc antibody. The density of the mature and immature forms of NKCC2 proteins was normalized to the density at time 0. Error bars represent S.E.
