FIGURE 7.
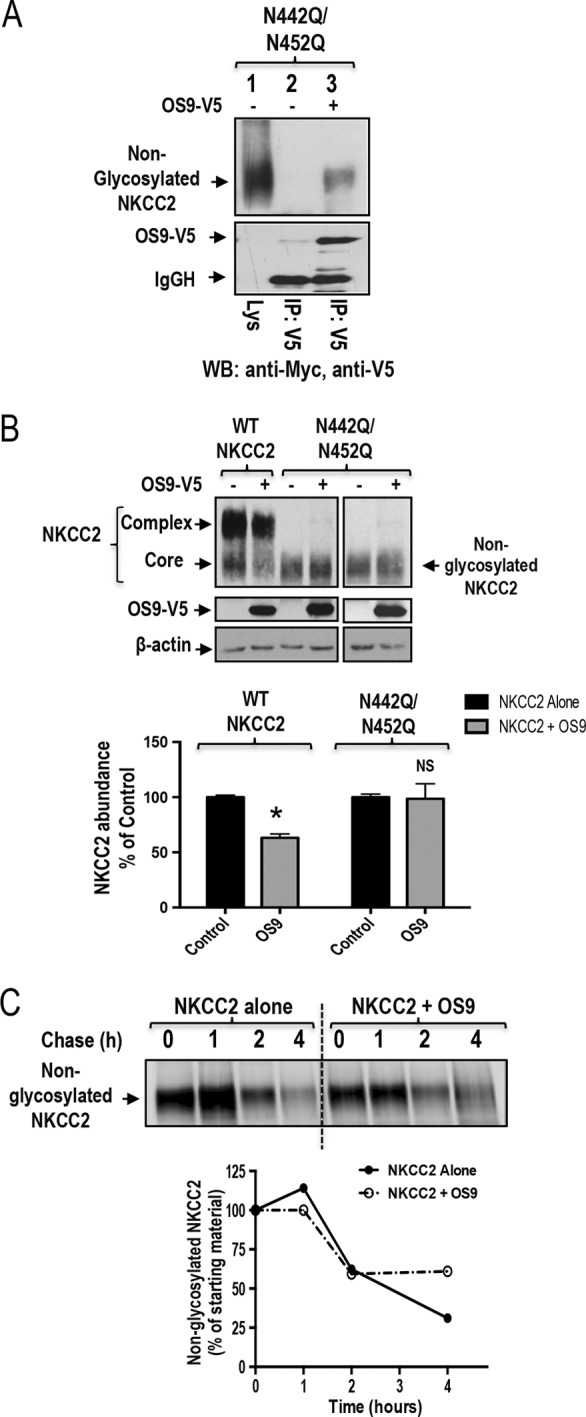
N-Glycosylation of NKCC2 is critical for the OS9-induced down-regulation of NKCC2. A, co-immunoprecipitation of OS9 with non-glycosylated NKCC2 (N442Q/N452Q). Cell lysates from cells transfected with N442Q/N452Q in the presence or absence of OS9-V5 were immunoprecipitated (IP) with anti-Myc or anti-V5 antibody. N442Q/N452Q co-immunoprecipitated with OS9 was detected by immunoblotting (WB) using anti-Myc (lane 3). B, OS9 association with non-glycosylated NKCC2 is unproductive. Cells were transfected with Myc-NKCC2 or N442Q/N452Q in the presence or absence of OS9-V5. 48 h post-transfection, total cell lysates were subjected to immunoblotting analysis for Myc-NKCC2, OS9-V5, and actin. Lower panel, quantitation of steady-state total NKCC2 expression levels with or without OS9 co-expression. *, p < 0.001 versus control (n = 4); NS, not significant versus control (n = 4). C, pulse-chase experiments performed in OKP cells transfected with non-glycosylated NKCC2 in the presence or absence of OS9 construct. Cells were labeled with [35S]methionine/cysteine and harvested at the indicated chase times for Myc-NKCC2 immunoprecipitation. Signals were detected by autoradiography. Lower panel, quantitative analysis of non-glycosylated NKCC2. The density of NKCC2 proteins was normalized to the density at time 0 (100%). IgGH, the heavy chain of IgG. Error bars represent S.E.
