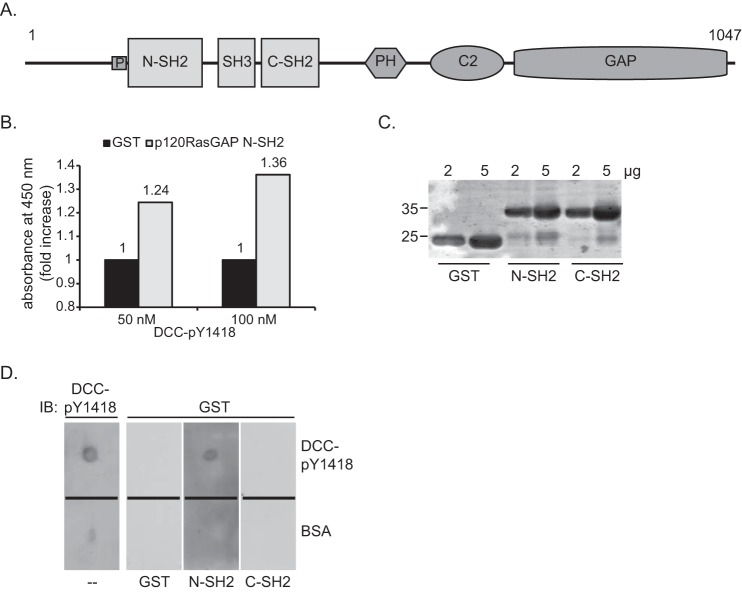
FIGURE 1.
The N-terminal SH2 domain of p120RasGAP interacts in vitro with DCC via phosphorylated Tyr-1418. A, p120RasGAP contains a proline-rich region (P) and N-SH2 and C-SH2, SH3, pleckstrin homology (PH), calcium-dependent phospholipid-binding (C2), and GAP domains. B, DCC- Tyr(P)-1418 was used as bait to screen a SH2 domain array by ELISA. Binding of p120RasGAP N-SH2 with 50 and 100 nm of DCC- Tyr(P)-1418 peptide is represented as the -fold increase in absorbance relative to the absorbance obtained with a GST control. C, 2 and 5 μg of purified GST, GST-p120RasGAP N-SH2, and C-SH2 were resolved by SDS-PAGE, and the proteins were stained with Coomassie Blue. D, the DCC- Tyr(P)-1418 peptide was spotted onto nitrocellulose membranes with BSA as a control, and each membrane was incubated with either purified GST, GST-p120RasGAP N-SH2, or C-SH2 (100 ng/ml), followed by immunoblotting (IB) with anti-GST antibodies. One membrane was immunoblotted with phospho-specific anti-DCC-pY1418 (DCC-pY1418) antibodies.