FIGURE 3.
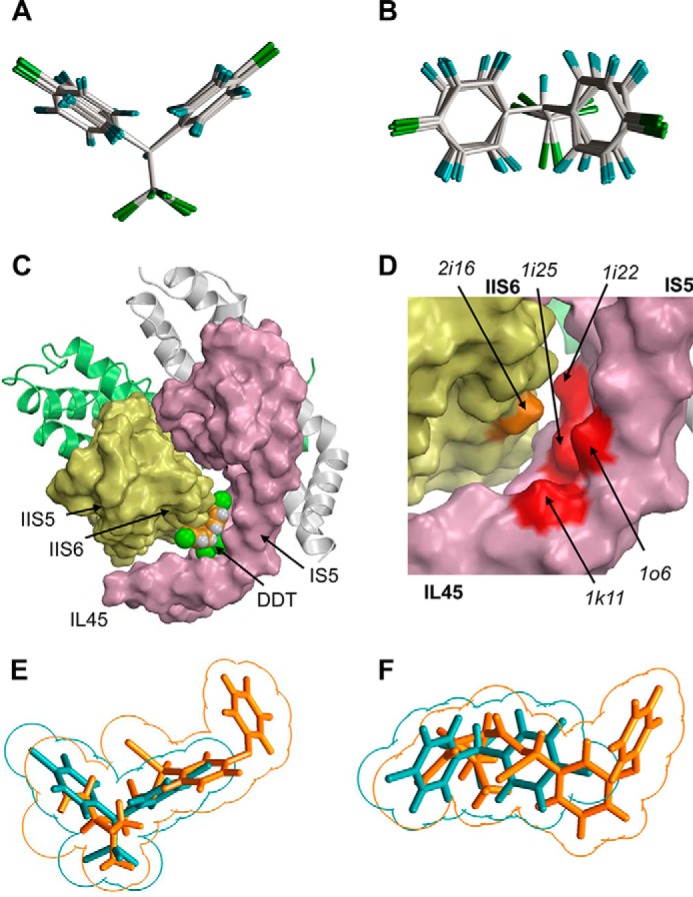
Predicting shape-complementarity complexes between DDT and the reduced x-ray structure of Kv1.2. A and B, orthogonal views of the global minimum conformation of DDT and local minimum conformations (within 7 kcal/mol from the global minimum). C, surface image of the x-ray structure of the open Kv1.2 channel in which all residues, except glycines, alanines, and prolines, are replaced with alanine to show potential ligand-binding clefts. The DDT molecule fits snugly into the interface between IL45, IS5, and S6. D, enlargement of C with the DDT molecule removed to show highlighted surfaces of some residues that contribute to the DDT-binding pocket. E and F, orthogonal views of the superimposed DDT (cyan) and deltamethrin (orange) molecules. Note an approximate size/shape similarity between the bulky hydrophobic dimethylcyclopropyl and C-CCl3 groups, leftward moieties bound to these groups, and similar angles at which two fragments of each ligand extend from of the bulky groups.
