FIGURE 7.
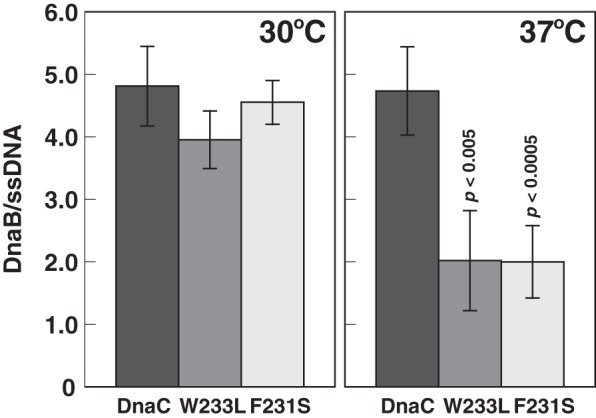
F231S and W233L are thermolabile in helicase loading. DnaA, DnaB, and DnaC were assembled onto M13 A site ssDNA at 30 or 37 °C. A portion of the sample equivalent to a standard assay to measure DNA replication was supplemented by the addition of primase, DNA polymerase III*, the β clamp and other required components at amounts used in a standard assay prior to isolation of the complex by gel filtration chromatography (Sepharose CL-4B; Pharmacia) as described under “Experimental Procedures.” The activity of the complex after isolation was also measured after adding required proteins and other components as described above to 20 μl of void volume fractions. The stoichiometry of DnaB, which represents the ratio of the DnaB-DnaC complex bound to the ssDNA, was then determined by quantitative analysis of the isolated complex. The mean and standard deviation from six or four independent analyses of isolated complexes from three or two experiments incubated at 37 or 30 °C, respectively, are shown in which the background ratios of nonspecifically bound DnaB per ssDNA in the absence of DnaA has been subtracted. These ratios ranged from 0.04 to 0.57. Student's t test analysis (two-tailed, two-sample equal variance) of the reduction at 37 °C compared with 30 °C yield p values of <0.005 for W233L and <0.0005 for F231S.
