FIGURE 1.
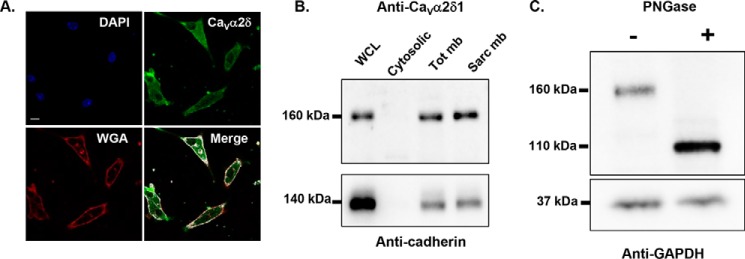
A, endogenous CaVα2δ1 proteins in mouse cardiomyocytes are glycoproteins. Endogenous CaVα2δ1 in 24-h cultured mouse cardiomyocytes co-localized with wheat germ agglutinin 647 (WGA 647), a plasma membrane marker that displays a high affinity for glycoproteins. CaVα2δ1 proteins were stained with the anti-CaVα2δ1 as the primary antibody and Alexa 488-coupled secondary antibody. Scale bar corresponds to 10 μm. The red channel was arbitrarily assigned to WGA, and the green channel was assigned to CaVα2δ1. Nuclei are stained with DAPI (blue). Co-localization pixel maps of CaVα2δ1 and WGA are shown in white and were produced using the co-localization finder plugin in FIJI. B, endogenous CaVα2δ1 proteins in the sarcolemmal membrane fraction of mouse cardiomyocytes migrate at 160 kDa. Four different protein fractions (total (WCL), cytosolic (Cyto), total membrane (Tot mb), and sarcolemmal membrane (Sarc mb)) were isolated from ventricles of adult CD-1 mice (50). Proteins were electrophoresed on an 8% SDS-polyacrylamide denaturating gel, transferred to a nitrocellulose membrane, and probed with an anti-CaVα2δ1 (Aviva System Biology). The membrane was probed, after stripping, with anti-pan-cadherin (Invitrogen 1:5000) as a quality control for the fractionation process. It is worth noting that the 160-kDa protein is the dominant species in whole-cell lysates. Each lane was loaded with 10 μg of proteins. C, PNGase F-mediated deglycosylation of the N-linked sugars in endogenous CaVα2δ1 proteins from mouse cardiomyocytes. Total whole-cell proteins isolated from ventricles of adult CD-1 mice were denatured 20 min at 60 °C before incubation in the absence (−) or presence (+) of PNGase F during 1 h at 37 °C. Each lane was loaded with 20 μg of proteins. Proteins were electrophoresed on an 8% SDS-polyacrylamide denaturing gel, transferred to a nitrocellulose membrane, and probed with an anti-CaVα2δ1 (Alomone Labs) (top panel) and anti-GAPDH (bottom panel) as a loading control.