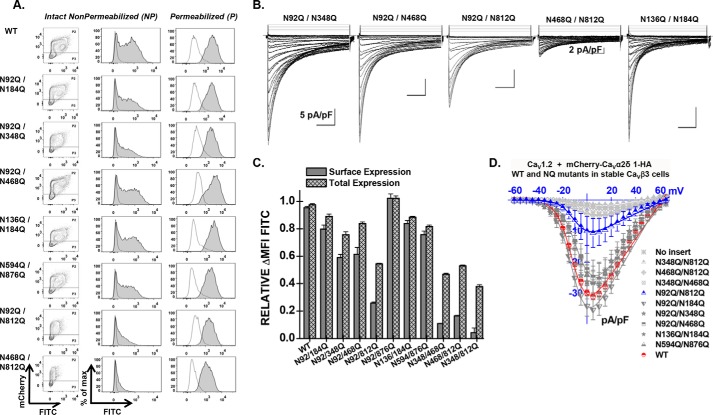
FIGURE 7.
Combining mutations N346Q, N468Q, and N812Q eliminates cell surface expression of CaVα2δ1 and modulation of CaV1.2 whole-cell currents. A, representative two-dimensional plots of mCherry versus FITC fluorescence are shown for each mutation as stated. B, representative whole-cell Ca2+ current traces recorded after recombinant expression of CaV1.2 in stable CaVβ3 cells with mCherry-CaVα2δ1-HA WT or some double N-glycosylation mutants. Unless specified otherwise, the current density scale is 5 pA/pF. Functional modulation by mCherry-CaVα2δ1-HA WT was measured under the same experimental conditions (data not shown). C, bar graph shows the normalized ΔMFI measured in the presence of FITC in intact (surface expression) or permeabilized cells (total expression). In all cases, the ΔMFI fluorescence measured for FITC in permeabilized cells was qualitatively similar to the constitutive fluorescence measured for mCherry validating the accessibility of the HA epitope and confirming the values obtained for total protein expression. D, current-voltage relationships, recorded in the presence of 2 mm Ca2+, are shown for the double mutations mCherry-CaVα2δ1-HA N92Q/N184Q, mCherry-CaVα2δ1-HA N92Q/N348Q, mCherry-CaVα2δ1-HA N92Q/N468Q, mCherry-CaVα2δ1-HA N92Q/N812Q, mCherry-CaVα2δ1-HA N348Q/N812Q, mCherry-CaVα2δ1-HA N468Q/N812Q, mCherry-CaVα2δ1-HA N348Q/N468Q, mCherry-CaVα2δ1-HA N594Q/N876Q, and mCherry-CaVα2δ1-HA N136Q/N184Q. See Tables 1 and 2 for details.