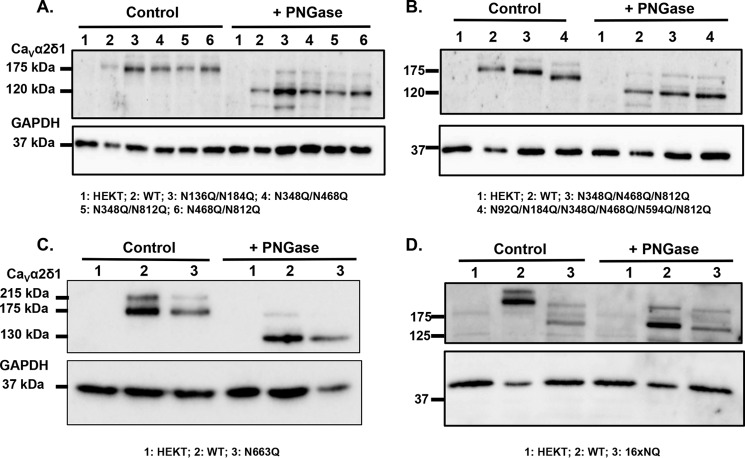
FIGURE 8.
Mutation of all 16 Asn sites appears to eliminate N-glycosylation from CaVα2δ1. HEKT cells were transiently transfected with pmCherry-CaVα2δ1-HA WT and mutants as described. One day after transfection, cells were lysed and protein lysates were either treated with the vehicle buffer (control conditions) or with PNGase F. Proteins were fractionated by SDS-PAGE (8%). Western blot analysis was carried out with the CaVα2δ1 antibody (Alomone Labs) as the primary antibody. A, lane 1, mock-transfected HEKT cells; lane 2, mCherry-CaVα2δ1-HA WT; lane 3, N136Q/N184Q; lane 4, N348Q/N468Q; lane 5, N348Q/N812Q; and lane 6, N468Q/N812Q. Before treatment with PNGase F, the calculated molecular masses for the high density band were as follows: lane 2, 173 kDa; lane 3, 163 kDa; lane 4, 163 kDa; lane 5, 166 kDa; and lane 6, 163 kDa. After digestion with PNGase F: lane 2, 123 kDa; lane 3, 121 kDa; lane 4, 121 kDa; lane 5, 119 kDa; and lane 6, 118 kDa. Lanes 2 (± PNGase) were loaded with 5 μg proteins and lanes 1; 3–6 (± PNGase) were loaded with 10 μg. B, lane 1, mock-transfected HEKT cells; lane 2, mCherry-CaVα2δ1-HA WT; lane 3, mCherry-CaVα2δ1-HA N348Q/N468Q/N812Q; and lane 4, mCherry-CaVα2δ1-HA N92Q/N184Q/N348Q/N468Q/N594Q/N812Q. Before PNGase F treatment, the calculated molecular masses for the high density band were as follows: lane 2, 173 kDa; lane 3, 159 kDa; and lane 4, 144 kDa. After digestion with PNGase: lane 2, 123 kDa; lane 3, 118 kDa; and lane 4, 116 kDa. Lanes 2 (± PNGase) were loaded with 5 μg of proteins, and lanes 1, 3, and 4 (± PNGase) were loaded with 10 μg. C, lane 1, mock-transfected HEKT cells; lane 2, pmCherry-CaVα2δ1-HA WT; and lane 3, N663Q. Before PNGase F treatment, the calculated molecular masses for the high density band were as follows: lane 2, 173 kDa; lane 3, 168 kDa. After digestion with PNGase F, the calculated molecular masses for the high density band were as follows: lane 2, 130 kDa; lane 3, 130 kDa. All lanes were loaded with 10 μg of proteins. D, lane 1, mock-transfected HEKT cells; lane 2, pmCherry-CaVα2δ1-HA WT; and lane 3, 16xNQ. Before PNGase F treatment, the calculated molecular masses for the high density band were as follows: lane 2, 183 kDa; lane 3, 132 kDa. After digestion with PNGase F, the calculated molecular masses for the high density band were as follows: lane 2, 130 kDa; lane 3, 127 kDa. Lanes 2 (± PNGase) were loaded with 10 μg of proteins, and lanes 1 and 3 were loaded with 20 μg. There was a 50-kDa reduction in the mobility of the recombinant CaVα2δ1 protein after the simultaneous mutation of the 16 Asn sites. Furthermore, enzymatic deglycosylation with PNGase F produced recombinant CaVα2δ1 proteins with the same apparent mobility suggesting that the 16 Asn sites account for the complete N-glycosylated state of the protein.