Abstract:
The purpose of this study was to describe the development and utilization of a perfusion quality improvement program to reduce perfusion-to-perfusion variability in a large multi-center perfusion practice. Phase I of the study included the establishment of a perfusion database using standard spreadsheet format to serve multiple administrative functions including patient and procedure sequencing, predictive algorithms for yearly caseload, summary statistics, and inter-perfusionist comparison. The database used 236 separate variables, including demographic and clinical procedure-related categories. Forty of these variables are modifiable by perfusion interaction as established via protocol and algorithm. Phase II of the study used a perfusion electronic data recording system to automatically obtain patient data from physiologic monitors and the heart-lung machine. Data were transferred to a central database for perfusionist comparison. Data analysis used logical functions and macros programming, and statistical analysis used both parametric and non-parametric models within the program. Each quarter all variables underwent analysis with summary data established for the most recent 225 patients undergoing CPB. Twenty-five cases from each perfusionist (n = 9) were compared with the aggregate data of the entire staff, with reference to previous quarter’s summary statistics. The results were discussed in monthly staff meetings and methods for improving compliance were discussed. Individual variation (p < .01) varied in 17 of 40 variables (26.0 ± 8.6), with quarterly improvement (27.4 ± 2.3 vs. 24.2 ± 2.1 vs. 17.0 ± 2.1) demonstrated in seven of nine individuals. In Phase II, performance was analyzed using the same variables as in Phase I but it also included the electronically recorded data from which 27 core measures were derived. All results were discussed with the staff at monthly departmental quality improvement meetings. The perfusion quality improvement program has evolved from a simple descriptive listing of cases to a quantitative instrument used to reduce variability amongst perfusionists and assure compliance with policies and standards of care.
Keywords: quality improvement, quality performance indicators, perfusion electronic medical record
Quality in medicine has become the central theme that guides the conduct of all aspects of patient care, and is predicated on processes that are formulated on consistency and reproducibility. Evidence exists that demonstrate an increase in clinical performance when quality initiatives are followed (1,2), and likewise, a decline when they are absent or poorly structured (3). One of the seminal components for improving quality is the incorporation of a metric system of performance that can be rigorously evaluated through quantitative analysis. The fields of cardiac surgery and cardiovascular perfusion are especially noteworthy for they use a large relatively homogeneous patient population that undergoes a consistent treatment that provides a basis for statistical review and evaluation. Indeed the surgical procedures of coronary artery bypass grafting and isolated valve surgery provides a template for the application of clinical trials on a wide variety of interventions and treatments.
Methods to review clinical performance are predicated upon fundamental tenets that include the development of large centralized databases, which generate several opportunities for improving care and enhancing safety. These include establishing a foundation for center-to-center comparison where the results of individual hospitals and practitioners can be compared with overall observed results (4), developing guidelines and standards created by task-forces and then endorsed by professional societies (5), and providing an ongoing review of local hospital-to-hospital performance. Quality is seen as an indication on how an organization is performing, and can be used as an incentive for patients in the selection of healthcare facilities. Likewise, third-party payers may use the results of a facilities performance as a means of awarding contracts and for paying for procedures when best practice guidelines are followed. Regulatory agencies are mandating that health-care facilities use quality as a core measure and incorporate the facets of quality improvement as a systematic process that drives the entire system. The Center for Medicare and Medicaid Services is developing a set of pay-forperformance initiatives to support quality improvement in the care of Medicare beneficiaries (Available at www.cms.hhs.gov/apps/media/press/release.asp?Counter=1343; accessed July 21, 2009). It is clear that performance measures have developed into touchstones by which the success of clinical enterprises is being gauged.
The majority of efforts in improving quality have been focused at a macro level examining large databases searching for relationships amongst covariates that identify influential variables that affect outcome (6). However, it is at the institutional level where performance is more readily modifiable to achieve the ultimate goal of improving patient care. A critical feature of methods directed at improving clinical conduct is the application of informatics technologies used for data acquisition and processing. Such an infrastructure will provide the basis for a coordinated and systematic review of how quality measures are influencing practice. The use of an electronic medical record (EMR) has gained such critical importance that many countries have mandated national incorporation with specific deadlines for its implementation (7,8). In per-fusion, the use of electronic data acquisition has long been touted as a tool for improving quality (9–11), although its acceptance by perfusionists has been slow, and its utility used primarily as a replacement to episodic written records (12). The real benefit of EMR lies in the incorporation of information into quality improvement processes where the electronically accumulated data will be used as an assessment for both individual and institutional performance. Such a process will ascertain the benefit of utilizing best practice guidelines shown to improve outcomes, and where lacking, to generate new knowledge based upon the results obtained when protocols are followed.
The purpose of this study was 2-fold: 1) To describe the development of a quality improvement process for standardizing performance across a multi-institutional per-fusion practice, and 2) To use a cardiopulmonary bypass (CPB) electronic data system (EDS) for data capture and for the evaluation of perfusionist adherence to algorithm and protocols as a tool for performance assessment.
MATERIALS AND METHODS
The study was approved by the Geisinger Health System (GHS) Institutional Review Board. The GHS is a large rural conglomerate of hospitals and clinics located in Northeastern Pennsylvania, with cardiac surgery performed at two of those facilities. (At the time of this study, Geisinger Health Systems consisted of three hospitals performing cardiac surgery: Geisinger Medical Center, Geisinger Wyoming Valley, and Geisinger South Wilkes Barre. Since that time the Geisinger South Wilkes Barre cardiac surgery program has merged with the Geisinger Wyoming Valley cardiac surgery program.) The perfusion department consists of nine individuals who have privileges at all three centers. All perfusionists are performing CPB on adult patients while a subgroup of perfusionists (4 of 9) conducts pediatric CPB. Therefore, only data from adult cases were included in this study. Despite having six adult cardiac surgeons there is only slight variability amongst clinicians as a result of an embracement of standardized care for cardiac patients (13). (The Proven Care Process described in this publication is primarily used for patients undergoing isolated coronary surgical revascularization.) Beginning in 2002, perfusionists at GHS systematically reviewed perfusion related techniques that had been shown, or purported, to improve outcomes of patients undergoing CPB. The review process was predicated on an evidence-based approach to ranking the quality of data for deciding on which was the best technique and methodology for all aspects of CPB. All information was obtained from the integrated text-based search and retrieval system managed by the United States National Library of Medicine (only English language citations were retrieved) (14). From these discussions, consensus agreement was used to determine which techniques and interventions would optimize the conduct of CPB. During this same period the health system was purchasing new heart-lung machines and ancillary perfusion equipment (centrifugal cell washing autotransfusion devices, in-line and extrinsic monitoring systems) for its centers. Since little data exists to show the superiority of one commercial heart-lung machine over another, the selection of equipment was based on two mandates: the requirement for intrinsic safety systems and the inclusion of an integrated perfusion EDS.
To determine which technologies and techniques would be incorporated, staff perfusionists were assigned specific subject areas for review. Assessment of published literature was made using techniques for classifying information according to established quality standards (15). Evidence was accepted at either the Class 1 or 11 recommendation criterion and at level A or B. All evidence was reviewed and consensus agreement reached before the technology or technique was accepted. Where appropriate, algorithms were developed that guided clinician conduct, and were established in the policy and procedure manual for the department. The cardiac surgeons were involved in these steps and approved all aspects of perfusion care delivery. Results of these efforts have been reported elsewhere and serve as the foundation for the quality improvement process (16). The entire process has been termed the Incremental Perfusion Improvement Process.
Integral to the development of Incremental Perfusion Improvement Process was the establishment of a database for recording variables associated with each clinical procedure, and is termed Phase I of the study. (Although the database includes both adult and pediatric patients only those patients greater than 19 years of age were included in this study.) The database was formed using spreadsheet format (Microsoft Excel, Microsoft Corp., Redmond, WA), and consisted of 236 variables. These were subdivided into eight major categories (demographics, hematocrit, autotransfusion, autologous prime, volumes, medications, cardiopulmonary bypass, and transfusion), and six subcategories. From this 90 variables were identified for comparison and used for analysis (see Appendix 1). Data was collected on standard perfusion records that included a preoperative patient work sheet and perfusion medical record, with additional data obtained from the electronic medical record (EPIC Version IU.2, Epic Systems Corp., Verona, WI), clinical pathology laboratory, and anesthesia record. The perfusion staff member assigned to the case was responsible for acquiring the data and for loading it into the perfusion quality program. Accuracy of the data was completed by a series of probability statements embedded into the spreadsheet that set limits around expected responses. When equations detected values that were outside of an expected range the program responded with a prompt to “Check Your Work". These equations worked by assuming numeric values that normally trended in a specific direction (i.e., a drop in hematocrit from the pre-CPB value to on-CPB) that failed to do so, and were most likely the result of an entry that was outside of an expected range. The perfusionist would then recheck the loaded values for accuracy. Once the entire data sheet was populated the program would signal that data was complete with a prompt of “OK.” All data was stored on a secure password protected computer and archived on a server, which was backed up each evening in the GHS mainframe. The database could be accessed from any computer across the three hospitals conducting cardiac surgery.
Phase II of the study began with the utilization of a perfusion EDS (Data Management System (DMSTM) Stockert® , Munich, Germany). The DMSTM is a data collection device that integrates a software package (DatabahnTM , ECC Online and ECC Server, Stockert®, Munich, Germany) with a computer and various bedside monitors capturing physiologic and mechanical information. The computer uses both keyboard and touch screen elements for data entry. The DMS has the potential to connect six serial devices through RS-232 connections to its hub, with one of the devices being the heart-lung machine (SIII TM, Stockert® , Munich, Germany). Data is automatically collected continuously but archived into the dataset at 20-second intervals. In the event of a system failure or power outage, the DMSTM stores the data to the computer hard drive once each minute. New patient information is loaded prior to each procedure starting except during emergent procedures. Here the DMSTM is activated with data capture occurring immediately, whereas patient background information (demographics, laboratory, personnel, etc.) is entered at a later time. During standard procedures data entry begins when the thoracic surgical incision is made and continues uninterrupted till the postprotamine activated clotting time sample has been completed. In the event of reinitiating CPB following after this time the DMS TM is reactivated and continued till the protamine has been readministered and activated clotting time corrected. Harvested data is then transferred wirelessly to a server where it is securely archived with automatic back-ups occurring several times throughout the day.
Data is collected from the following devices: SIII TM heart-lung machine (Stockert®, Munich, Germany), Myocardial Protection System TM (MPS, Quest Medical Inc.®, Allen, TX), CDI 500 Blood Gas Monitor TM (Terumo Cardiovascular Inc.®, Ann Arbor, MI), INVOS Cerebral Oximeter TM (Somanetics Corp.®, Troy, MI), and a patient bedside monitor (IntelliVue MP 90 TM , Philips Corp.® , Bothell, WA).
In addition to the parameters collected manually, and listed in Appendix 1, the utilization of the EDS provided for additional variable data collection. The variables included continuous data from the heart-lung machines as well as peripheral devices and are shown in Appendix 2. From this data a series of performance indicators has been devised that use the captured information from the EDS. These data are then used to calculate performance parameters that are listed in Table 1 .
Table 1. Calculated values from data captured by the perfusion electronic data system.
| INVOS percent time | <40% |
| INVOS percent right > lLeft | >10% |
| Percentage MAP | <50 mmHg (mmHg*min) |
| Flow rate time (min) | <.25 Cardiac Index |
| Percentage of time | <1.8 Cardiac Index |
| Percentage of time | >2.6 Cardiac Index |
| Percentage of time line pressure | >300 mmHg |
| Percentage of time venous > arterial temperatures | |
| Percentage of time arterial > patient temperature | >6°C |
| Percentage of time | >37°C |
| Percentage of time VAVD | >−40 mmHg |
| Percentage of time pHa | <7.35 |
| Percentage of time pHa | >7.45 |
| Percentage of time PaCO2 | <35 mmHg |
| Percentage of time PaCO2 | >45 mmHg |
| Percentage of time PaO2 | <150 mmHg |
| Percentage of time PaO2 | >250 mmHg |
| Percentage of time hematocrit | <22% |
| Area under the curve of hematocrit | <22% |
| Change in hematocrit from postinduction to first CPB | |
| Change in hematocrit from postinduction to last CPB | |
| VO2 high and low | |
| Percentage of time SVO2 | <60% |
| Area under the curve of SVO2 | <60% |
| Percentage of time BE | <−2 |
| Area under the curve of BE | <−2 |
| Total percentage of events when |
BE, base excess; MAP, mean arterial pressure; pHa, pH arterial; PaCO2, partial pressure of carbon dioxide in the arterial blood; PaO2, partial pressure of oxygen in arterial blood; SVO2, mixed venous oxygen saturation; VO2, oxygen consumption.
Following the establishment of the perfusion quality improvement program, data was collected for 15 months as a prologue to Phase I. During this trial time information was regularly reviewed by the perfusion staff and modifications and revisions made to the database. Phase I was conducted from April through December of 2007. Phase II data began in 2008 and consists of all first-quarter (January through March) data.
STATISTICS AND REVIEW
All data was analyzed on a quarterly basis. Each perfusionist had the most recent 25 cases summarized using descriptive statistics. A master dataset of the most recent 225 cases was created and used as the population to which each perfusionist individual data was compared. Data was analyzed using both non-parametric (Chi Square, Wilcoxon Rank Sum) and parametric analysis (Student’s t test), and statistical significance accepted at the p < .05 level and trending towards significance accepted at p < .10 level. The entire group discussed results in ensuing staff meetings where each perfusionist had their data reviewed during quality improvement meetings. Quarterly results were tabulated and sequentially reviewed to track performance. For comparative purposes data from each quarter in a calendar year were compared to data only within that year. Data is presented as mean, standard deviation, median, maximum, minimum, and 1st and 3rd quartiles.
RESULTS
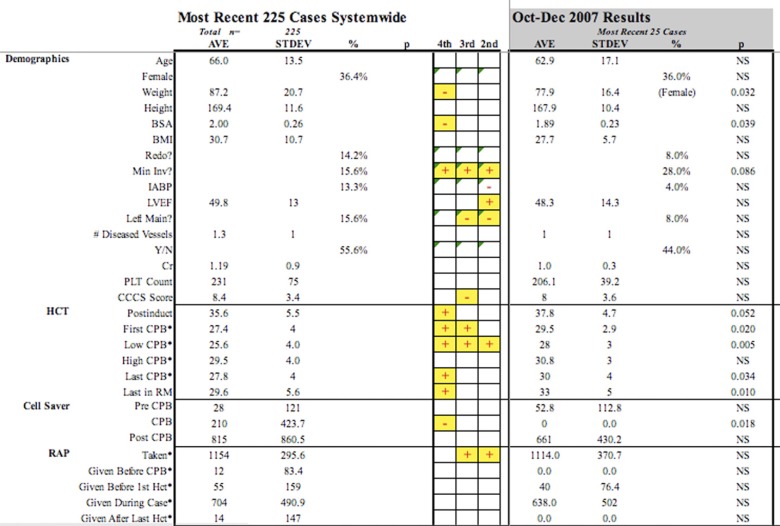
Of the 90 variables used for performance comparison, 40 are identified as being modifiable by perfusion actions or interventions. The mean, standard deviation, and percent occurrence were compared amongst perfusionists and benchmarked to the previous 225 patients. The study period lasted from March through December of 2007 with each perfusionist producing three quarters of data for comparison with a total of 27 time periods amongst perfusionists. An example of the quarterly report is shown in Figure 1.
Figure 1.
Graphic depiction of several parameters contained in quality improvement quarterly report. Negative (?) marks indicate that the perfusionist quarterly data was statistically less than the 225 aggregate data, while positive (+) marks indicate statistically higher results.
Summing the statistical differences within each variable across all perfusionists and dividing that number by the total quarters expressed variation amongst results. Patient size, as delineated by height, weight, body surface area, and body mass index, varied 3.7%, 25.9%, 18.5%, and 22.2% respectively.
The categories deemed most dependent upon perfusion intervention were Hematocrit, Autologous Prime, and Volumes and are shown in Table 2. Baseline hematocrit values, identified as post induction and measured prior to any surgical incision, had a total variation of 14.8% amongst perfusionists across periods, whereas the on-CPB hematocrit variation ranged from 37–55%.
Table 2.
Distribution of variation in performance across major
| 2nd Quarter | 3rd Quarter | 4th Quarter | |
|---|---|---|---|
| Categories | |||
| Demographics | 21 | 20 | 16 |
| Hematocrit | 18 | 19 | 23 |
| Autotransfusion | 2 | 3 | 4 |
| Autologous Prime | 9 | 9 | 6 |
| Volumetrics | 21 | 17 | 17 |
| Medications | 9 | 4 | 3 |
| Cardiopulmonary Bypass | 26 | 27 | 32 |
| Transfusion | 8 | 10 | 14 |
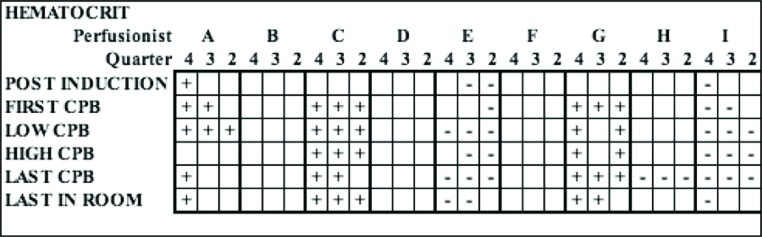
Two perfusionists (C and G) had significantly higher hematocrit values across all quarters at 93% and 80% despite having the same post induction starting hematocrit as the entire group (Figure 2). Two perfusionists (E and I) had hematocrit values significantly lower than the aggregate values across 73.3% and 80% of all CPB time periods, but three of the six post induction periods showed lower starting hematocrits. Autologous prime techniques showed only minor variation amongst the entire group. However, two perfusionists were able to achieve statistically higher autologous prime volume removal in two of three quarters, while one perfusionist was significantly lower across all quarters with volume removal.
Figure 2.
Graphic depiction of single parameter (hematocrit) across quarters and amongst perfusion staff. Negative (−) marks indicate that the perfusionist quarterly data was statistically less than the 225 aggregate data, while positive (+) marks indicate statistically higher results. CPB, cardiopulmonary bypass.
In the Volumes category there were 11 comparative indicators for each perfusionist during each quarter for a total of 33 over the study period. The variation in volume administration ranged from a low of 18.2% to a high of 60.6%. Seven of the nine staff perfusionists had significantly lower administration of crystalloid and colloidal volumes in 30% of all variables when compared with the aggregate total, but two perfusionists had significantly higher volumes in greater than 50% of the time periods. Two perfusionists were also found to have a significantly higher utilization of 25% albumin in the prime solution, and one staff member had significantly higher utilization of red blood cells than all other perfusionists in each of the three quarters.
In Phase II a total of 162 variables directly harvested from the DMS were analyzed along with 27 performance indicators that were derived from the captured values. (In between Phase I and Phase II there was a loss of one staff perfusionist.) Aggregate results for core measures are shown in Table 3.
Table 3.
Cardiopulmonary bypass performance indicators derived from perfusion electronic data system.
| Performance Core Measures | Aggregate % |
|---|---|
| INVOS percent time <40% | 1.7 ± 4.3 |
| INVOS percent right > left >10% | 7.9 ± 19.0 |
| Percentage MAP <50 mmHg (mmHg*min) | 11.5 ± 8.9 |
| Flow rate time (min) <.25 cardiac index | 1.1 ± 1.3 |
| Percentage of time <1.8 cardiac index | 18.6 ± 21.9 |
| Percentage of time >2.6 cardiac index | 9.3 ± 22.0 |
| Percentage of time line pressure >300 mmHg | 15.0 ± 26.8 |
| Percentage of time venous > arterial temperatures | 1.3 ± 1.8 |
| Percentage of time arterial > patient temperature >6°C | 10.0 ± 17.3 |
| Percentage of time >37°C | 4.0 ± 7.9 |
| Percentage of time VAVD >-40 mmHg | 6.4 ± 20.3 |
| Percentage of time pHa <7.35 | 24.9 ± 25.4 |
| Percentage of time pHa >7.45 | 7.8 ± 16.5 |
| Percentage of time PaCO2 <35 mmHg | 5.0 ± 9.3 |
| Percentage of time PaCO2 >45 mmHg | 36.1 ± 29.6 |
| Percentage of time PaO2 <150 mmHg | 1.6 ± 2.4 |
| Percentage of time PaO2 >250 mmHg | 30.1 ± 25.0 |
| Percentage of time hematocrit <22% | 6.1 ± 19.0 |
| Area under the curve of hematocrit <22% | 22.4 ± 70.0 |
| Change in hematocrit from postinduction to first CPB | 7.7 ± 4.6 |
| Change in hematocrit from postinduction to last CPB | 7.7 ± 5.0 |
| Highest VO2 | 213.1 ± 58.0 |
| Percentage of time SVO2 <60% | .3 ± .8 |
| Area under the curve of SVO2 <60% | 6.7 ± 32.9 |
| Percentage of time BE <−2 | 4.1 ± 11.7 |
| Area under the curve of BE <−2 | −10.9 ± 28.1 |
| Total percentage of events when protocol not followed | 3.0 ± 1.2 |
BE, base excess; MAP, mean arterial pressure; pHa, pH arterial; PaCO 2, partial pressure of carbon dioxide in the arterial blood; PaO2 , partial pressure of oxygen in arterial blood; SVO 2, mixed venous oxygen saturation; VO2, oxygen consumption .
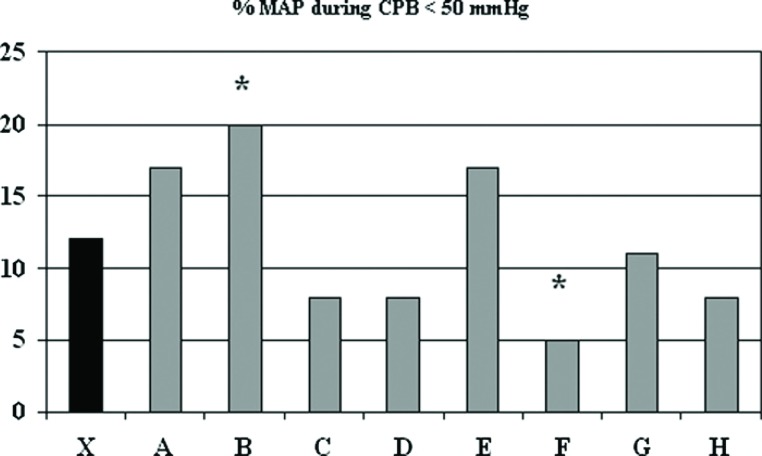
For the first quarter of 2008 a total of 57 patients undergoing CPB were entered into the perfusion quality database and analyzed. The only preoperative demographic parameter that differed amongst perfusionists was a lower BSA for perfusionist C’s patients (1.82 ± .15 vs. 2.04 ± .28, p < .05) compared with the mean of the entire population. There were a total of 376 discrete cells during the CPB period that were used for performance comparison and of those, 27 (7.2%) were significantly different than the mean values for all perfusionists. Twenty-four of the 27 cells were a result of actions modifiable by perfusion intervention, and 10 (2.7%) were deemed negative deviations from the accepted algorithms and policies established at GHS. One perfusionist (B) had significantly lower mean arterial pressure (<50 mmHg) during CPB (B) compared to the group, while one staff member (F) had significantly lower threshold pressure than the group (Figure 3).
Figure 3.
Percent of time that mean arterial pressure during cardiopulmonary bypass was less than 50 mmHg. The mean (X) indicates results for most recent 225 procedures and letters designate individual perfusionists. CPB, cardiopulmonary bypass; MAP, mean arterial pressure.
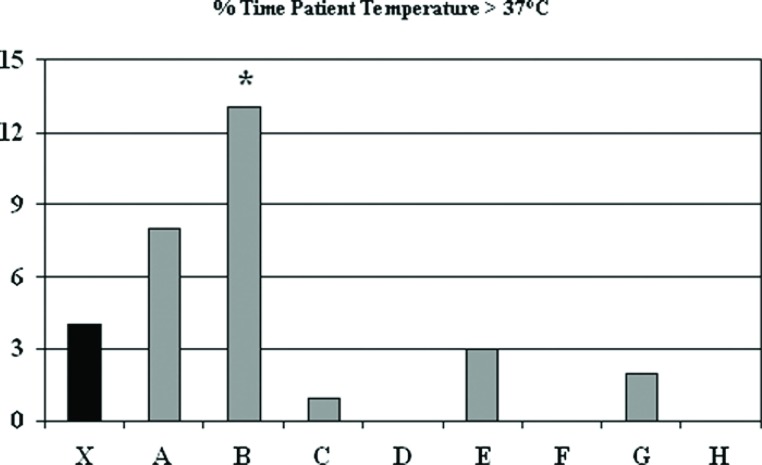
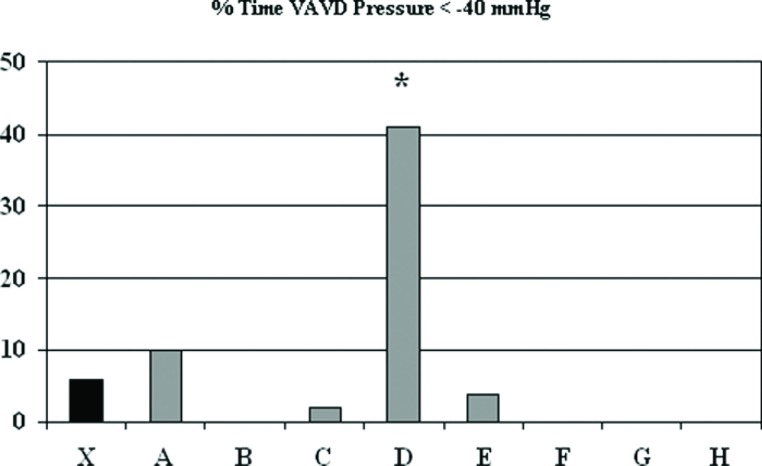
The GHS policy for warming is not to exceed a threshold value of 37°C arterial perfusate temperature. Figure 4 shows that the majority of the staff were able to achieve this greater than 97% of the time, but one perfusionist (B) had significantly higher temperatures than the aggregate mean (13.0 ± 10.1 vs. 4.0 ± 7.9%, p < .05). The protocol for use of vacuum assist venous drainage (VAVD) was set at an upper limit of negative pressure of ?40 mmHg. This was exceeded 6.4 ± 20.3% of the time and one perfusionist (D) had lower VAVD pressures 41 ± 47% of the time (p < .001) (Figure 5).
Figure 4.
Percent of time that patient temperature during cardiopulmonary bypass was greater than 37°C. The mean (X) indicates results for most recent 225 procedures and letters designate individual perfusionists.
Figure 5.
Percent of time that vacuum assist venous drainage pressure during cardiopulmonary bypass was less than −40 mmHg. The mean (X) indicates results for most recent 225 procedures and letters designate individual perfusionists. VAVD, vacuum assist venous drainage.
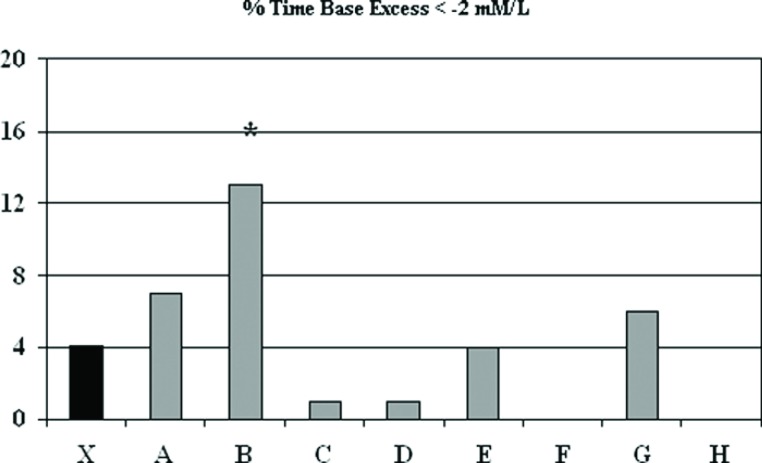
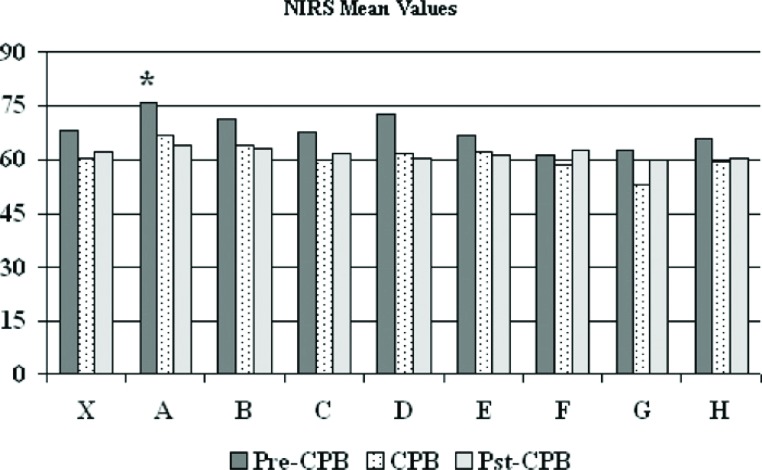
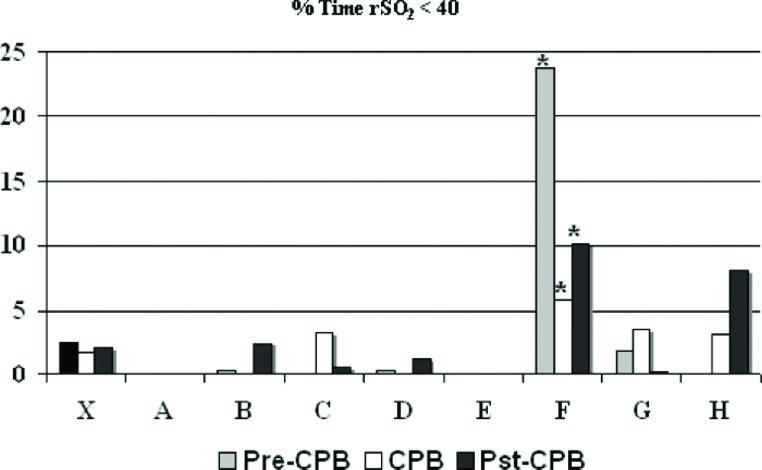
Base excess was maintained at ?2 to +2 mM/L and was exceeded 4.1 ± 12.7% of the time, with one perfusionist (B) higher than the group aggregate (12.6 ± 24.5 vs. 4.1 ± 11.7%, p < .09) (Figure 6). Cerebral oximetry using near infrared spectroscopy was assessed and is shown in Figures 7 and 8. Near infrared spectroscopy mean values decreased only slightly from pre-CPB to on-CPB and the post-CPB time periods. One perfusionist showed a significantly reduced regional cerebral saturation (rSO2) value of <40 compared with aggregate numbers, but there was also lower rSO2 found in the pre-CPB and post-CPB periods as well (Figure 8). Results for all other CPB related variables showed occasional variation amongst perfusionists but deviation from accepted protocols established at GHS was absent.
Figure 6.
Percent of time that base excess during cardiopulmonary bypass was less than −2 mmol. The mean (X) indicates results for most recent 225 procedures and letters designate individual perfusionists.
Figure 7.
The near infrared spectroscopy mean values during cardiopulmonary bypass. The mean (X) indicates results for most recent 225 procedures and letters designate individual perfusionists. CPB, cardiopulmonary bypass; NIRS, near infrared spectroscopy.
Figure 8.
Percent of time that regional oxygen saturation during cardiopulmonary bypass was less than 40%. The mean (X) indicates results for most recent 225 procedures and letters designate individual perfusionists. CPB, cardiopulmonary bypass; rSO2, regional oxygen saturation.
DISCUSSION
During the past decade the healthcare industry has placed tremendous emphasis on the importance of quality improvement as a guiding force for organizational conduct (17). All healthcare facilities are mandated to develop processes that improve the quality of care they provide to patients. Regulatory organizations provide operating guidelines that hospitals follow to achieve compliance and be recognized with accreditation and certification. However, these guidelines are often enhanced within institutions by supplemental directives aimed at improving safety with concomitant effects on care. Regulatory agencies that have incorporated quality and safety guidelines for healthcare organizations include the Joint Commission (18), the Centers for Medicare and Medicaid Services (19), and the National Quality Forum (20). All of these organizations possess a central theme of promoting strategies that emphasize the measuring and reporting of results directly related to patient care. A central tendency of such structure is the reduction in variation both within and amongst healthcare organizations.
Cardiac surgical procedures are particularly attractive for statistical evaluation because of their wide degree of application, similarity in surgical treatment, and comprehensive national and regional databases for the collection of case-specific information (21). Data obtained from these reporting systems has been used to establish benchmarks for both hospital and physician comparisons. The ultimate goal is the reduction in variability whereby lower achieving facilities review their practice techniques and processes to improve their performance.
In America many state governments mandate that their health departments collect statistics from hospitals performing cardiac surgery. Such tracking is purported as a monitoring device for the comparison of outcomes, which has led to the development of “report cards” comparing both hospital and physician results. Although the exact benefit of such reporting is debated, and the accuracy of data questioned, it does draw attention to performance issues, which may affect referral patterns to those facilities and physicians.
For perfusionists, no federal or state reporting mechanisms exist so that efforts to improve care through data analysis are done within departments or within perfusion service companies. Although the electronic collection of perfusion data has been described for quite some time, few reports appear in the literature that show how variability within perfusion departments or organizations is reduced. Those that do exist are mainly focused at broad practice variables that may not be consistent with approaches to care that have been linked to strong evidence for their benefit. Baker and Newland have shown that utilization of a perfusion EDS can reduce variability amongst staff through a quality improvement process that use an automated feedback mechanism to alert staff to changes from protocol (22). Variability reduction models such as Statistical Process Control Theory are well established in business and technology areas to improve productivity and reproducibility. The application of these theories in medicine has gained popularity during the past decade as a means of reducing both hospital and clinician variability, and Groom et al. have editorialized their potential for application in perfusion (23).
Efforts to reduce clinical variability have not always been viewed as positive. The utilization of algorithmic driven processes for applying care, such as clinical or critical pathways, can be deemed as lowering the clinician’s ability to intercede by reducing personal judgment and experiential knowledge, and has been viewed as a “cookbook style medicine” approach (24). Quantitative analysis of the role that human judgment plays in clinical decision making is difficult primarily due to the fact that individual practitioners vary in their decision making abilities. Furthermore, medical judgment requires that clinicians maintain a guarded skepticism and use a conservative approach to accepting new techniques or technologies that require change. For this reason the standardization of healthcare delivery should include a human element that remains central to the encounter with each patient, but should not supercede the application of evidence based guidelines when they exist. For the past decade our center has embraced the former concept and used a “proven care” approach to the conduct of both CPB and cardiac surgery (13,15).
The field of perfusion quality assurance has had a long history, but was primarily developed more as a safety perspective (25,26), to neither reduce variability nor improve outcomes. More recently, Newland and associates from Adelaide, Australia have shown how data mined from the perfusion EMR can be incorporated into a quality assurance program that provided close to real time feedback on performance (11,12,22). This innovative technique demonstrates one of the most sought after and pertinent benefits of electronic data management systems in perfusion. Riley and associates were amongst the first to describe the use of microprocessors to record data from various physiological instruments in a laboratory setting representing one of the first efforts for the electronic recording of perfusion data (9,10). Jegger et al. developed a simple assessment tool for performance evaluation amongst perfusionists (27). They compared several common perfusion parameters across established ranges using a scoring system for performance. Although the data was entered manually, as previously described problematic, these researchers used a team approach using surgeons, perfusionists, and anesthesiologists in the decision making process. More recently, this same research team developed a scoring mechanism to show that certain CPB parameters and using both multivariate and univariate analysis linked them to outcome data (28). Although this study was not randomized nor controlled, it does demonstrate a plausible effort to relate perfusion interventions to outcomes. Similarly, Dickinson et al. used the results published by entities that track hospital cardiac surgical outcomes based upon certain quality indicators that can then be used to benchmark hospitals across a region (29). The authors then compared data of several quality indicators, routinely collected by staff from a commercial perfusion organization that provided services to top hospitals as well as hospitals not on either list, and found that in the top hospitals the perfusion staff consistently performed higher than in matched facilities.
Initially data collection was facilitated using a manual entry mode. Such a system, while effective for processes that do not consist of a large number of variables or data entry units, is ineffective for CPB (12). The construct of a quality improvement program that relies on individuals for manually entering data has inherent flaws. These include assessing the accuracy of collected data, and minimizing human factors that may influence data entry when performance criteria have been established. However, such systems are commonly encountered in many perfusion programs that use data management systems. However, these systems and the utilization of databases can serve as a useful foundation for transitioning to an electronic system for quality improvement. This is what was accomplished in Phase I of the present study and was helpful in guiding the development of methodologies for Phase II.
Electronic data collection does not mean that invalid data could not be collected. Indeed automation of data collection often leads to a new set of problems since the systems collect all events regardless of their accuracy. This has been one of the major drawbacks to their inclusion in American perfusion practices where medical-legal issues are more sensitive, but may be less so in other areas of the world less so encumbered (personal communication with product managers of cardiopulmonary equipment manu facturers who provide an electronic perfusion medical record). Regardless, the incorporation of invalid data into a performance improvement program would undermine the effectiveness of the system and have the opposite effect on staff morale and more than likely lower motivation for self-improvement. We have incorporated a mechanism for assessing the accuracy of the data by establishing limits for the range of captured data. This is done automatically when data is transferred from the DMS to the spreadsheet by the creation of logical function.
In the present study, our goal was to use a quantitative analysis approach in determining how the perfusionists across the GHS were performing. This followed a multiyear process whereby all technologies used for CPB were reviewed and evaluated using a preemptive scientific evidence-based approach for selection and incorporation into practice (30). For interventions where a lack of evidence was present or discrepancies in the literature were found, the judgment of the perfusion and cardiac surgery team members was ascertained. Group discussions on technologies and techniques not only enabled consensus agreement but also had the additional benefit of fostering collegiality and empowering staff as decision makers. This process continues to date and is viewed as a continuum for quality improvement without termination or cessation. We found that communication was critical in assuring that all clinicians had voice in commenting upon the technologies and initiatives concerning perfusion care. The entire clinical team benefited by the system of structured inclusiveness as well as the seminal ownership of decisions.
Although the greatest reduction in perfusion variation occurred during the pilot phase, (first 15 months of data collection), it continues to date, albeit with smaller reductions occurring during the latter part of the trial. This is not unrealistic since drawing attention to performance by metric analysis and measurement has long been shown as a critical element of variability reduction. However, the assessment of performance in the field of perfusion has had only a limited degree of success, which can be related to a number of elements. First, there have been reports that the quality of evidence to support the selection of one perfusion technique over another is lacking, which hinders change or the adoption of new methods (31). Second, the absence or lack of clinical trials in perfusion technology has created a void of evidence that, although identifies opportunities for the creation of new knowledge, makes the assessment of new technologies difficult. The establishment of organizations such as the International Consortium on Evidence-Based Perfusion (32) has as their core mission the goal of addressing these shortcomings. Third, perfusionists tend to be independently minded practitioners, as many healthcare workers are, who guard their autonomy carefully making the acceptance of standards for perfusion challenging. Furthermore, the level of education of perfusionists varies greatly across the world, which, when combined with economic constrictions for equipment utilization, adds geography as an independent variable for the perfusion conduct. And lastly the manufacturers of electronic perfusion medical records have been slow to address the full utility of the potential for quality improvement. This has required perfusionists to develop their own database systems and incorporate statistical methodologies themselves. Few centers have the resources to place into the establishment of perfusion data analysis systems. However, most hospitals do have information technology departments that will become more sophisticated as the national mandate for the establishment of a universal EMR becomes a reality. These resources will be available to healthcare facilities in America, as well as those abroad that have similar initiatives, as a means of improving patient care. Perfusionists will benefit from the establishment of such an infrastructure and are educationally and technically prepared to excel in performance enhancement.
From this research it becomes quite clear that the ultimate goal of perfusion quality improvement programs is to incorporate outcome data for each procedure into perfusion databases on a patient-by-patient basis. The availability of national databases such as that of the Society of Thoracic Surgeons, is a tremendous resource that has exportable data from each institution that can link the information obtained from the perfusion EDS to patient outcome. Once this is done it would be relatively easy to analyze data lesion-specific so that perfusion conduct could be assessed both from an historical perspective as well as prospectively. Opportunities for the conduct of perfusion research on a multi-institutional level would be achievable where both techniques and technologies could be assessed, and the creation of new knowledge for the establishment of recommendations made.
In conclusion, the establishment of a perfusion quality improvement program has resulted in a reduction in overall clinical performance variation. Such a program has created a system for motivation that has improved the conduct of perfusion, which has been formulated on an evidence-based approach for the improvement of care.
APPENDIX 1: PERFUSION QUALITY PARAMETERS FROM PERFUSION DATABASE
Demographics
Age
Gender
Weight
Height
Body Surface Area
Body Mass Index
Resternotomy
Minimally Invasive Procedures
Intra-aortic Balloon Pump (preoperative, intraoperative, postoperative)
Left Ventricular Ejection Fraction
Left Main Disease
Number of Coronary Artery Diseased Vessels
Cleveland Clinic Clinical Severity Score
Bernstein/Parsonnet 2000 Risk Score
PREOPERATIVE LABORATORY VALUES
Creatinine
Blood Urea Nitrogen
Platelet Count
Hematocrit
HEMATOCRIT
Postinduction
First CPB
Low CPB
High CPB
Last CPB
Last in Room
Autotransfusion
Autotransfusate returned (Pre-CPB, During CPB, Post-CPB)
Cardiopulmonary Bypass
-
Autologous Prime (Volume)
Taken Prior to CPB
Given Before CPB
Given Before First Hematocrit Measurement
Given During Case
Given After Last Hematocrit Measurement
Volumetrics
-
Pre-CBP
Albumin (5 and 25%)
Crystalloid
Packed Red Blood Cell (units)
Urine Output
-
CPB
Prime Crystalloid
Albumin (5 and 25%)
Crystalloid
Packed Red Blood Cell (units)
Fresh Frozen Plasma (units)
Crystalloid Cardioplegia
Other Volumes
Ultrafiltration (Zero Balance, Modified)
Urine Output
-
Post-CPB
Crystalloid
Albumin (5 and 25%)
Crystalloid
Packed Red Blood Cell (units)
Fresh Frozen Plasma (units)
Urine Output
-
Time
CPB
Cross Clamp
Fibrillation
Circulatory Arrest
-
Cardioplegia
Antegrade Administration
Retrograde Administration
Number of Doses
Longest Interval
Hot Shot
Substrate Enhanced Warm Reperfusate
Cardioversion Required
-
Anticoagulation
Baseline Activated Clotting Time (ACT)
Heparin Dose
Additional Doses of Heparin
Pre-CPB ACT
First CPB ACT
High CPB ACT
Low CPB ACT
Last CPB ACT
Additional CPB Heparin
Protamine Dose
Post Protamine ACT
Additional Protamine
Post Additional; Protamine ACT
Medications
Neosynephrine
Heparin
NaHCO3 (bicarbonate of soda)
Norepinephrine bitartrate
Aprotinin
Amicar
-
Protamine
AT III
Prothrombin Complex Concentrate
Transfusion Requirements
Any Transfusion
Total Packed Red Blood Cells (units)
Total Fresh Frozen Plasma (units)
Total Platelet (units)
Total Cryoprecipitate (units)
APPENDIX II: PERFUSION QUALITY PARAMETERS TAKEN FROM ELECTRONIC DATA SYSTEM
Demographics
Age
Gender
Weight
Height
Body Surface Area
Body Mass Index
Blood Type
RH
Pre-CPB Arterial Blood Gas
pHa (pH arterial)
PaCO2 (partial pressure of carbon dioxide in the arterial blood)
PaO2 (partial pressure of oxygen in arterial blood)
HCO3 (bicarbonate) BE (base excess)
SaO2 (saturation of oxygen)
Glucose Na+ (sodium ion)
K+ (potassium ion)
Ca++ (serum calcium)
Monitoring
-
Hemodynamics
Arterial Mean Blood Pressure
Pulmonary Artery Mean Pressure
-
Temperatures
Nasophyrangeal
CPB Arterial
CPB Venous
-
Cerebral (INVOS, SomaneticsTM)
Right Side
Left Side
Cardiopulmonary Bypass
Arterial Blood Flow Rate
Cardiac Index
Arterial Line Circuit Pressure
Vacuum Assisted Venous Drainage Pressure
-
In-Line Blood Gas Monitor (CDI 500TM , Terumo Cardiovascular)
pHa
PaCO2 (partial pressure of carbon dioxide in the arterial blood)
HCO3 (bicarbonate)
BE
SaO2 (saturation of oxygen)
Temperature
SvO2 (mixed venous oxygen saturation)
K+ (potassium ion)
Hemoglobin
Hematocrit
Activated Clotting Time
CPB Time
Cross Clamp Time
Microbubble Event Count
Volumetrics
Volume In
Volume Out
Crystalloid Solutions
Albumin (25%)
Albumin (5%)
Ultrafiltration
Modified Ultrafiltration
Urine
Packed Red Blood Cell (units)
Fresh Frozen Plasma (units)
Cryoprecipitate (units)
Platelets (units)
Medications
Neosynephrine
Heparin
NaHCO3 (bicarbonate of soda)
Levophed
Vasopressin
Protamine
REFERENCE
- 1.Hosford SB.. Hospital progress in reducing error: The impact of external interventions. Hosp Top. 2008;86:9–20. [DOI] [PubMed] [Google Scholar]
- 2.Miller MR, Pronovost P, Donithan M, et al. Relationship between performance measurement and accreditation: Implications for quality of care and patient safety. Am J Med Qual. 2005;20:239–52. [DOI] [PubMed] [Google Scholar]
- 3.Longo DR, Hewett JE, Ge B, Schubert S.. Hospital patient safety: Characteristics of best-performing hospitals. J Healthc Manag. 2007;52:188–204. [PubMed] [Google Scholar]
- 4.Jacobs JP, Mavroudis C, Jacobs ML, et al. Nomenclature and databases—The past, the present, and the future: A primer for the congenital heart surgeon. Pediatr Cardiol. 2007;28:105–15. [DOI] [PubMed] [Google Scholar]
- 5.Grover FL, Shroyer AL, Hammermeister K, et al. A decade’s experience with quality improvement in cardiac surgery using the Veterans Affairs and Society of Thoracic Surgeons national databases. Ann Surg. 2001;234:464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyse RK, Taylor KM.. Using the STS and multinational cardiac surgical databases to establish risk-adjusted benchmarks for clinical outcomes. Heart Surg Forum. 2002;5:258–64. [PubMed] [Google Scholar]
- 7.Etheredge LM.. Technologies of health policy. Health Aff (Millwood). 2007;26:1537–8. [DOI] [PubMed] [Google Scholar]
- 8.Rynning E.. Public trust and privacy in shared electronic health records. Eur J Health Law. 2007;14:105–12. [DOI] [PubMed] [Google Scholar]
- 9.Riley JB.. A technique for computer assisted monitoring in the management of total heart-lung bypass. J Extra Corpor Technol. 1981;13:171–6. [Google Scholar]
- 10.Riley JB, Hurdle MB, Winn BA, Wagoner PA.. Automation of cardiopulmonary bypass data collection. J Extra Corpor Technol. 1985;17:7–12. [Google Scholar]
- 11.Newland RF, Baker RA, Stanley R.. Electronic data processing: The pathway to automated quality control of cardiopulmonary bypass. J Extra Corpor Technol. 2006;38:139–43. [PMC free article] [PubMed] [Google Scholar]
- 12.Ottens J, Baker RA, Newland RF, Mazzone A.. The future of the per-fusion record: Automated data collection vs. manual recording. J Extra Corpor Technol. 2005;37:355–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Casale AS, Paulus RA, Selna MJ, et al. ProvenCareSM: A provider-driven pay-for-performance program for acute episodic cardiac surgical care. Ann Surg. 2007;246:613–21. [DOI] [PubMed] [Google Scholar]
- 14.US National Library of Medicine, National Institutes of Health. Pubmed. Available at: www.ncbi.nlm.nih.gov/sites/entrez/db= pubmed. [Google Scholar]
- 15.Shahian DM, Edwards FH, Ferraris VA.. Quality measurement in adult cardiac surgery: Part 1—Conceptual framework and measure selection. Ann Thorac Surg. 2007;83:S3–12. [DOI] [PubMed] [Google Scholar]
- 16.Trowbridge CC, Stammers AH, Wood GC, et al. Improved outcomes during cardiac surgery: A multifactorial enhancement of cardiopulmonary bypass techniques. J Extra Corpor Technol. 2005;37:165–72. [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Medicine. Performance Measurement: Accelerating Improvement. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 18.The Joint Commission. Available at: www.jointcommission.org. [Google Scholar]
- 19.The Centers for Medicare and Medicaid Services. Available at: www.cms.hhs.gov. [PubMed] [Google Scholar]
- 20.The National Quality Forum. Available at: www.qualityforum.org. [Google Scholar]
- 21.Grover FL, Shroyer ALW, Hammermeister K, et al. A decade’s experience with quality improvement in cardiac surgery using the Veterans Affairs and Society of Thoracic Surgeons national databases. Ann Surg. 2001;234:464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker RA, Newland RF.. Continuous quality improvement of perfusion practice: The role of the electronic data collection and statistical control charts. Perfusion. 2008;23:7–16. [DOI] [PubMed] [Google Scholar]
- 23.Groom R, Likosky DS, Rutberg H.. Understanding variation in cardiopulmonary bypass: Statistical process control theory. J Extra Corpor Technol. 2006;36:224–30. [PubMed] [Google Scholar]
- 24.Morris AH.. Computerized protocols and bedside decision support. Crit Care Clin. 1999;15:523–45. [DOI] [PubMed] [Google Scholar]
- 25.Eagle CJ, Davies JM, McCloskey B.. An introduction to quality assurance with an application for perfusionists. J Extra Corpor Technol. 1991;23:22–5. [PubMed] [Google Scholar]
- 26.Winn BA, Hurdle MB, Riley JB, et al. A quality assurance program for perfusion. J Extra Corpor Technol. 1986;18:138–9. [Google Scholar]
- 27.Jegger D, Revelly JP, Horisberger J, et al. A cardiopulmonary bypass score system to assess quality of perfusion performance. Perfusion. 2001;16:183–8. [DOI] [PubMed] [Google Scholar]
- 28.Jegger D, Ruchat P, Horisberger J, et al. Establishing an association between a peri-operative perfusion score system (PerfSCORE) and post-operative patient morbidity/mortality during CPB cardiac surgery. Perfusion. 2007;22:311–6. [DOI] [PubMed] [Google Scholar]
- 29.Dickinson T, Riley J, Zabetakis PM.. External validation of compliance to perfusion quality indicators. Perfusion. 2004;19:295–9. [DOI] [PubMed] [Google Scholar]
- 30.Holcomb H, Stammers AH, Nutter B, et al. Perfusion treatment algorithm: Methods of improving the quality of perfusion. J Extra Corpor Technol. 2003;35:290–6. [PubMed] [Google Scholar]
- 31.Bartels C, Gerdes A, Babin-Ebell J, et al. Cardiopulmonary bypass: Evidence or experienced based. J Thorac Cardiovasc Surg. 2002;124:20–7. [DOI] [PubMed] [Google Scholar]
- 32.International Consortium for Evidence Based Perfusion. Available at: www.bestpracticeperfusion.org. [Google Scholar]