FIGURE 6.
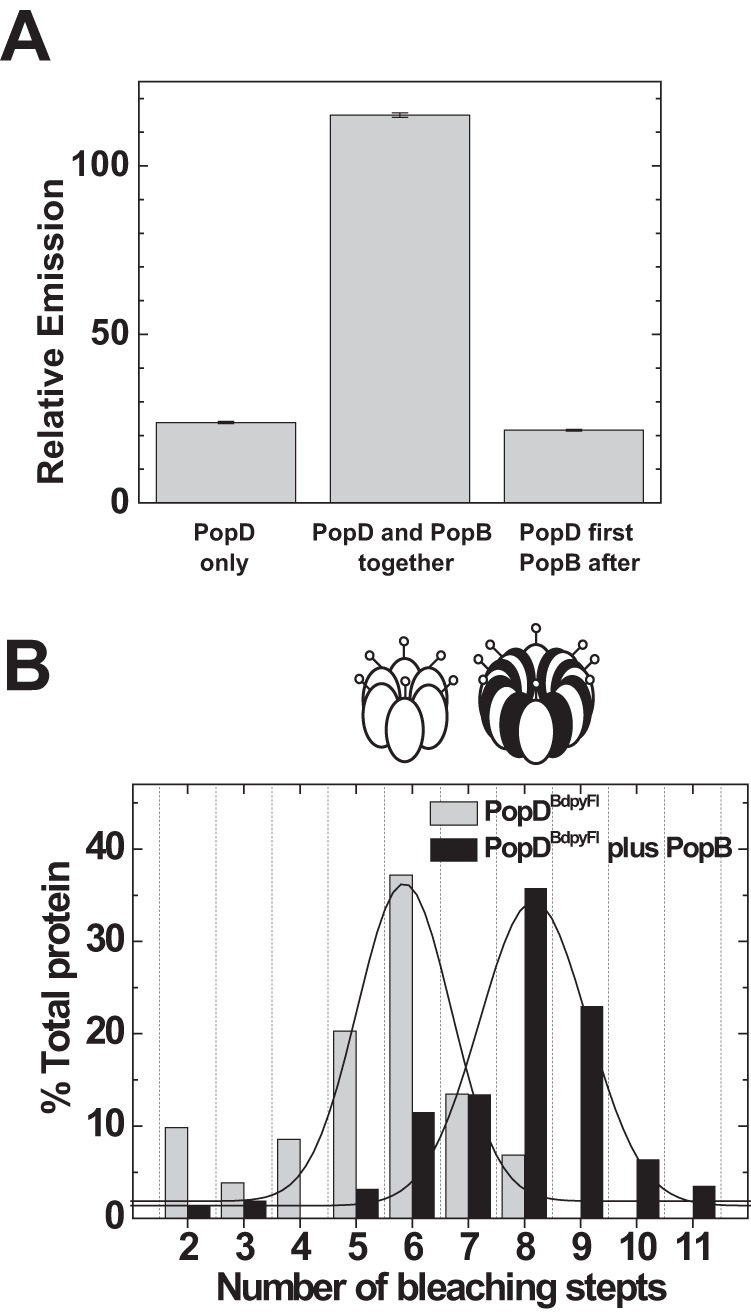
Simultaneous membrane interaction favored hetero-oligomerization over formation of translocator homo-oligomers. A, formation of the PopD homo-oligomer is not reversed by PopB addition to membranes. Left bar, PopDBpyFL was incubated with membranes and the final self-quenched emission determined (PopD only). Center bar, PopDBpyFL was mixed in 6 m urea buffer with a 10-fold excess of PopBWT and the mixture was added to membranes. Excess PopB favored the incorporation of PopD into hetero-oligomers, therefore it de-quenches the emission of the BpyFl probe (see also Fig. 3B). Right bar, PopDBpyFL was incubated with membranes to allow homo-oligomer formation and a 10-fold excess of PopB was subsequently added. No emission de-quenching was observed in this case, indicating that the simultaneous interaction with membranes of both translocators is necessary to form hetero-oligomers. A constant amount of PopDBpyFL (83% labeled with BpyFL) was present in every sample at a final concentration of 10 nm. The protein to lipid ratio was 1/6000 in all samples. Error bars indicate the range between two measurements. B, PopD size distribution shifts from 6 to 8 subunits per complex when membrane incubation is done in the presence of PopB. PopDBpyFL was incubated with membranes in the presence of 10-fold excess PopB whereas maintaining a constant protein:lipid ratio (black bars). Size distributions were obtained using single-molecule photobleaching and compared with the distribution obtained when PopDBpyFL was incubated alone with membranes (Fig. 4C, reproduced in gray bars). Lines indicate Gaussian fits to the histogram data. Oligomers are represented as coded in Fig. 3.
