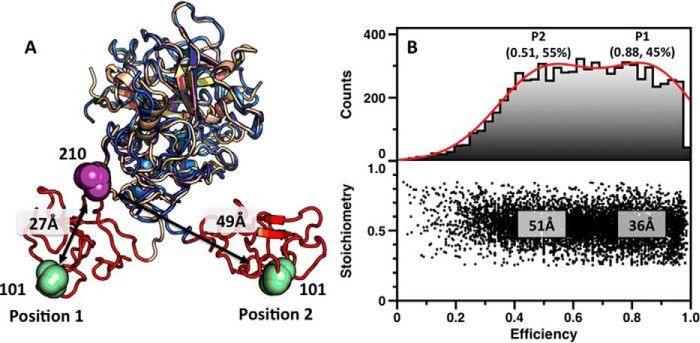
FIGURE 7.
Conformational flexibility of Lnk2 revealed by single molecule FRET. A, structures of ProTΔ146–167 (Protein Data Bank code 4O03, wheat; Protein Data Bank code 5EDK, blue), with the Gla domain removed for clarity. The two structures overlap significantly (RMSD = 0.56 Å) at the level of the kringle-2/protease domain pair but differ sharply in the orientation of kringle-1 (red). The change is due to the conformation of Lnk2 that switches from α-helix (Protein Data Bank code 4O03, position 1) to β-strand (Protein Data Bank code 5EDK, position 2). Residues Ser101 and Ser210 are represented as green and magenta spheres, with arrows indicating Cα-Cα distances. B, FRET histograms for the mutant ProTΔ146–167/S101C/S210C. The lower panel shows a two-dimensional histogram of stoichiometry (S) versus FRET efficiency (E) for each diffusing molecule that contains both AF555 and AF647 fluorophores (i.e. molecules with 0.25 < S < 0.75). The upper panel shows a one-dimensional E histogram of the molecules in the lower panel. The E distribution was fit to a double Gaussian distribution (red). The center and percentage of the population in each Gaussian is indicated. FRET efficiencies were converted to distances using a theoretical R0 = 51 Å and a random orientation factor k = 2/3.