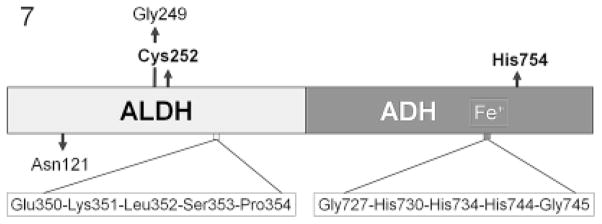
Fig. 7.
Schematic diagram of Entamoeba histolytica alcohol dehydrogenase 2 (EhADH2). The enzymatic activities reside in two separate, interacting domains: N-terminal aldehyde dehydrogenase (ALDH) and C-terminal alcohol dehydrogenase (ADH) (catalytic residues: Cys252 and His754, respectively). Conserved amino acids essential for each domain’s function are shown; the iron-binding region in ADH is crucial for Eh-ADH2 activity and interaction between domains (Espinosa et al. 2001, 2009).