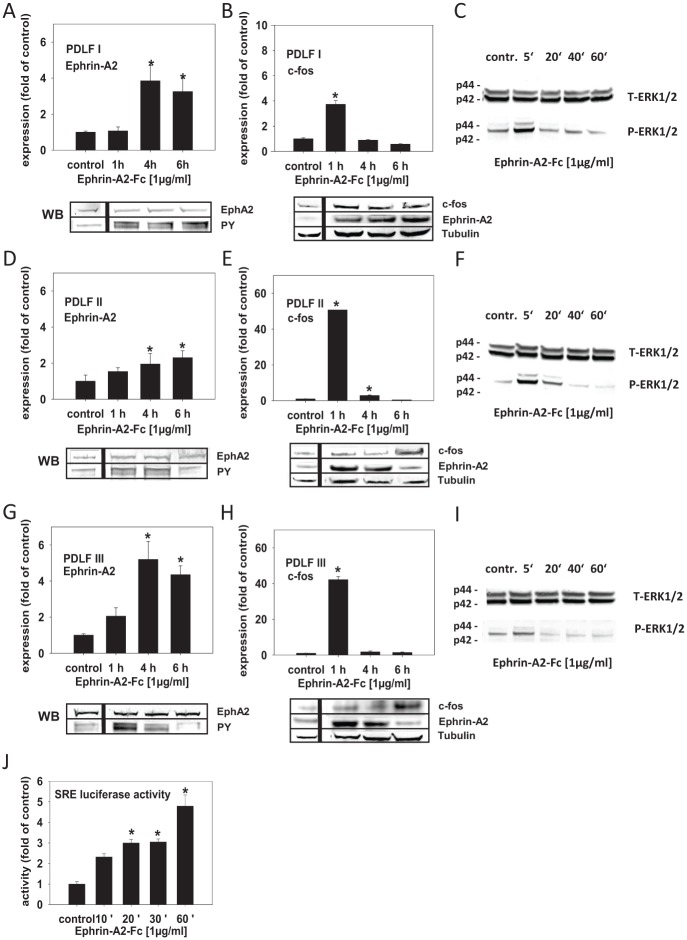
Figure 3.
Ephrin-A2 is involved in its own induction in compressed periodontal ligament fibroblasts (PDLFs). EphA2 stimulation by different ephrin-As was shown to promote the activation of ERK kinases, which, via ternary complex transcription factor (TCFs), could lead to the induction of serum response element (SRE), the pivotal cis-element involved in the regulation of the transcription of immediate early genes, including c-fos. Therefore, ephrin-A2 via ERK1/2 SRE activation might be involved in the regulation of c-fos, which could lead to its own transcriptional activation. To test this, PDLFs were stimulated with preclustered ephrin-A2–Fc and incubated for 1, 4, and 6 h. (A, D, G) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) showed that ephrin-A2–Fc significantly induced the transcription of ephrin-A2 in the 3 analyzed PDLF populations (PDLF I, II, and III) 4 h after stimulation; transcription remained enhanced until 6 h after stimulation. (B, E, H) qRT-PCR demonstrated that the activation of ephrin-A2 transcription was preceded by a significant, transient induction of c-fos 1 h after stimulation with ephrin-A2–Fc. (C, F, I) Ephrin-A2–Fc stimulation might activate ephrin-A2 transcription via ERK1/2 phosphorylation. Western blotting for ERK1/2 and pERK1/2 showed that ERK1/2 phosphorylation was evident in PDLFs 5 min after stimulation, returning to background levels after 20 min. To test for EphA2 receptor phosphorylation after ephrin-A2–Fc stimulation, immunoprecipitation of EphA2 and subsequent probing with an anti–phosphotyrosine antibody (PY) was performed (A, D, G, lower panel). The regulation of ephrin-A2 and c-fos after ephrin-A2–Fc stimulation was confirmed on the protein level (B, E, H, lower panel). Tubulin served as a loading control. (J) To test whether ephrin-A2–Fc stimulation leading to ephrin-A2 transcriptional activation involves the activation of SRE, a reporter construct (pSRE-Luc) was transfected into murine 3T3 fibroblasts. 3T3 cells were stimulated with preclustered ephrin-A2–Fc for 10, 20, 30, and 60 min. Enhanced SRE-dependent luciferase activity was measured 10 min after stimulation, reached significance after 20 min, and remained significantly upregulated until the end of the observation period at 60 min. Stimulation experiments were performed in triplicates (n = 3). Cells treated with the anti–Fc antibody (0.1 µg/mL), time matched, served as controls. Luciferase reporter gene assays were performed twice in triplicates. The qRT-PCR assays were performed in triplicates. Data are presented as mean ± SD. *P < 0.05 vs. control (one-way analysis of variance, Dunnett’s post hoc test).