Figure 7.
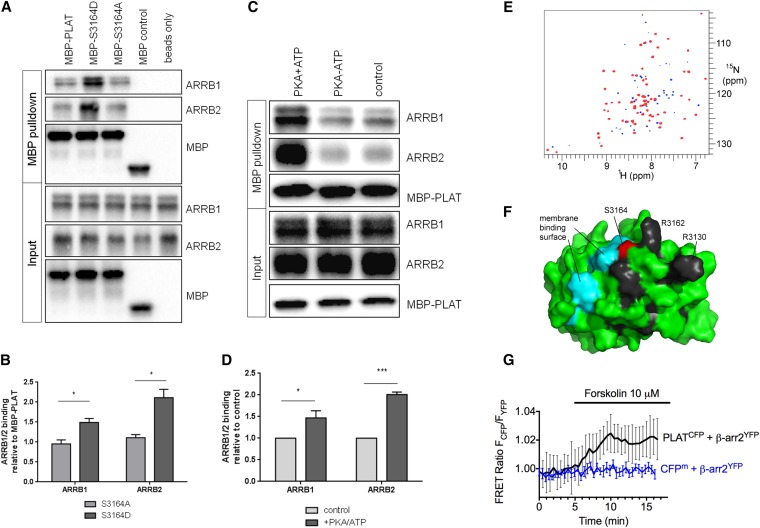
PKA phosphorylation of PLAT increases its binding affinity to ARRB1/2. (A and B) MBP-PLAT pulldown assays show that PLAT can bind to ARRB1 and ARRB2, and this interaction was significantly increased by 2-fold for the S3164D-PLAT MBP fusion compared with the wild type or S3164A-PLAT. Molecular mass of MBP is 43kD, MBP-PLAT is 57kD, His-ARRB1 is 49kD, and His-ARRB2 is 46kD, respectively. *P<0.05 versus S3164A-PLAT (n=3). (C and D) MBP-PLAT pulldown assays after PKA phosphorylation of PLAT. PLAT binding to ARRB1 and ARRB2 was significantly increased by 1.5- to 2-fold after PKA pretreatment of MBP-PLAT. *P<0.05; ***P<0.001 versus control MBP-PLAT (n=3). (E) TROSY HSQC spectra of 200 μM GB1-PLAT alone (blue) and in the presence of 1.5 equivalents ARRB1 (red; superimposed). Peaks with significantly reduced intensity in the presence of ARRB1 are blue. Residues K3125, G3129, R3130, H3161, R3162, G3177, Q3198, D3217, S3220, and V3234 show a clear progressive loss of intensity throughout the titration. (F) Mapping of these residues onto the surface of PLAT. The most strikingly affected residues are shown in black. Also shown are S3164 (red), the site of phosphorylation, and the region shown to interact with phosphatidyl serine (cyan), indicating that ARRB1 binds adjacent to S3164 and the membrane binding site. (G) Averaged time course of the association between PLAT-CFP and ARRB2-YFP in response to forskolin. FRET recordings are shown as normalized ratios and represent mean values±SEM (n=15). Addition of forskolin to a single cell increased the FRET signal by approximately 2%, with a time constant of t1/2=1.14 minutes. A membrane-anchored CFP (CFPm) served as a negative control.