Figure 4.
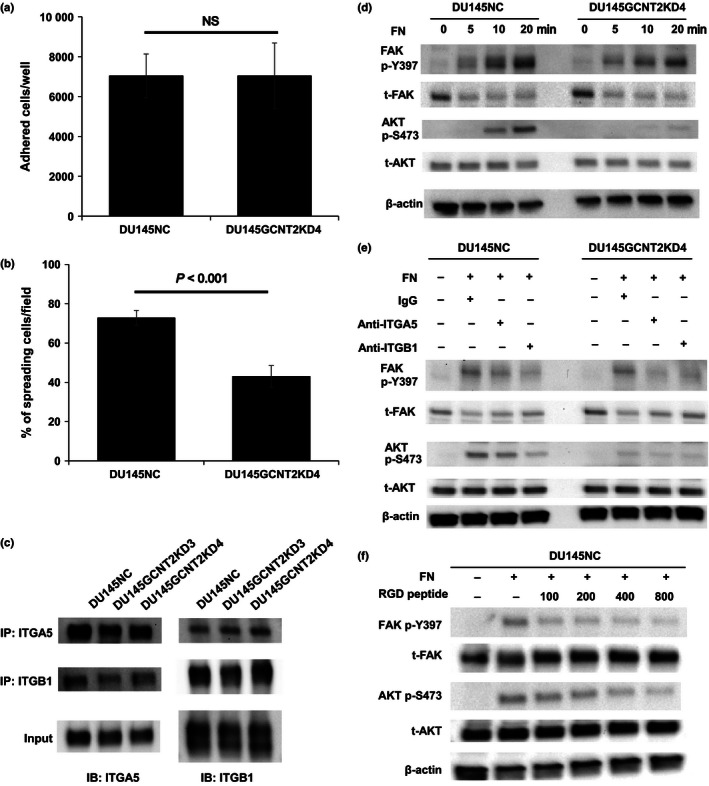
I‐antigen regulates cell spread and α5β1 integrin–fibronectin interactions that are mediated by protein kinase B (AKT) phosphorylation in DU145NC and DU145GCNT2KD4 cells. Cell adhesion and spreading on fibronectin (FN) was examined by harvesting cells after a 30 min of culture on fibronectin‐coated 96‐well plates (a). DU145NC and DU145GCNT2KD4 cells showed no significant differences in adhesion potential. (b) Cells were harvested from fibronectin‐coated glass slides after 30 min of incubation, and spreading cells were visualized using crystal violet solution. Percentages of spreading cells were significantly lower in DU145GCNT2KD4 cells than in DU145NC cells. (c) Cell lysates from DU145 cells were immunoprecipitated using anti‐integrin α5 or β1 antibodies, and heterodimers were detected. I‐branching N‐acetylglucosaminyltransferase (GCNT2) expression had no effect on heterodimerization of α5β1 integrin. (d) Focal adhesion kinase (FAK) and AKT are downstream targets of integrin signaling. FAK phosphorylation at tyrosine 397 (p‐Y397) was similar in DU145NC and DU145GCNT2KD4 after integrin–fibronectin interactions. In contrast, AKT phosphorylation at serine 473 (p‐S473) was strongly inhibited by GCNT2 knockdown in DU145 cells. (e) A functional blocking antibody against α5 and β1 integrins inhibited α5β1 integrin–fibronectin interactions. Pretreatment of DU145NC and DU145GCNT2KD4 cells with anti‐integrin α5 or β1 led to lower FAK p‐Y397 and AKT p‐S473 levels compared with those after pretreatment with IgG isotype control. (f) Pretreatment of DU145NC cells with RGD peptide (100, 200, 400, or 800 μM) inhibited FAK p‐Y397 and AKT p‐S473 in a concentration‐dependent manner. IB, immunoblotting; IP, immunoprecipitation; NS, not significant; t‐FAK, total‐FAK; t‐AKT, total‐AKT.
