Abstract
Purpose
GPA33 is a colorectal cancer (CRC) antigen with unique retention properties after huA33-mediated tumor targeting. We tested a pre-targeted radioimmunotherapy (PRIT) approach for CRC using a tetravalent bispecific antibody with dual specificity for GPA33 tumor antigen and DOTA-Bn (radiolanthanide metal) complex.
Methods
PRIT was optimized in vivo by titrating sequential intravenous doses of huA33-C825, the dextran-based clearing agent (CA), and the C825-haptens 177Lu-or 86Y-DOTA-Bn in mice bearing the SW1222 subcutaneous (s.c.) CRC xenograft model.
Results
Using optimized PRIT, therapeutic indices (TIs) for tumor radiation absorbed dose of 73 (tumor/blood) and 12 (tumor/kidney) were achieved. Estimated absorbed doses (cGy/MBq) to tumor, blood, liver, spleen, and kidney for single-cycle PRIT were 65.8, 0.9 (TI: 73), 6.3 (TI: 10), 6.6 (TI: 10), and 5.3 (TI: 12), respectively. Two cycles of PRIT treatment (66.6 or 111 MBq 177Lu-DOTA-Bn) were safe and effective, with 9/9 complete responses of established s.c. tumors (100–700 mm3) and 2/9 alive without recurrence >140 d. Tumor log kill in this model was estimated to be 2.1–3.0 based time to 500-mm3 tumor recurrence. In addition, PRIT dosimetry/diagnosis was performed by PET imaging of the positron-emitting DOTA-hapten 86Y-DOTA-Bn.
Conclusions
We have developed anti-GPA33 PRIT, as a triple-step theranostic strategy for pre-clinical detection, dosimetry and safe targeted radiotherapy of established human colorectal mouse xenografts.
Keywords: multistep targeting, bispecific antibodies, GPA33, radioimmunotherapy, pretargeting
INTRODUCTION
Despite therapeutic advances in the last decade, colorectal cancer (CRC) is still the fourth leading cause of death worldwide, and in 2015 49,700 CRC deaths are projected in the US alone [1]. Although locoregional therapies such as surgery and radiation can be curative in many cases, these approaches do not control tumors which have metastasized at diagnosis, where chemotherapy and targeted small molecules are most often palliative, with rapid development of resistance. Clearly, there is an unmet need for improved therapeutics, particularly for treatment of unresectable metastatic disease.
In principle, targeted radiation therapy strategies such as radioimmunotherapy (RIT) could potentially address this unmet need, because it enables delivery of tumoricidal radiation doses to multiple systemically dispersed sites simultaneously (e.g., ranging from macroscopic nodal or hepatic oligometastases to occult micrometastatic disease) as long as the therapeutic indices (TIs) are favorable [2]. Notably, the majority of RIT trials against CRC have been with IgGs directed at carcinoembryonic antigen (CEA) (e.g., 131I-hMN-14 [3] and 90Y-cT84.66 [4]); so far, these studies have enjoyed limited clinical success because of suboptimal TIs of only 5–10:1 for macroscopic tumors, which is inadequate for curative therapy without dose-limiting hematological toxicity.
Since particularly radioresistant solid tumors such as CRC may require a total absorbed dose of 7–10,000 cGy for cure, we need highly selective tumor targeting with respect to radiosensitive normal tissues (typically bone marrow, which may be suppressed by doses as low as 150 cGy). The RIT approach that offers the greatest potential for the required improvement in TI is the pretargeted approach (PRIT) utilizing bispecific antibodies (BsAb) [5]. For small volume or microscopic disease, there is evidence that uptake and consequently TIs may be higher both in animal models [6, 7] and human patients [8, 9]. This, taken in conjunction with the reduced tumor cell number in such small tumors, means that the likelihood of achieving complete ablation is expected to be greater than in the case of macroscopic disease, so long as a radionuclide appropriate for small volume disease is used [10]. However, in the most general case, small volume disease will co-exist with macroscopic disease in the same patients and treatment approaches will be required that address this entire disease spectrum. Moreover, if PRIT can improve the treatment of macroscopic disease, it will concomitantly improve that of microscopic disease by the same mechanisms and at no additional cost
PRIT, a concept that separates the delivery of the targeting antibody to tumor from the delivery of the radioactivity, was initially developed by Reardan, Goodwin, and Meares using anti-radiometal chelate antibodies [11–13], Initial uptakes documented proof of principle for this approach, showing improved tumor targeting compared to conventional RIT, but TIs were still suboptimal, partly because of the insufficient retention (on the order of several hours) of the radioactivity when carried by anti-chelate antibodies of only nanomolar (nM) affinity [14, 15].
The BsAb/radiometal binding approach of Reardan and colleagues took a major step forward with the development of ultra-high affinity antibodies for the radiometal-chelate binding step, based on work by Orcutt, Wittrup and colleagues at MIT. Guided by a mathematical modeling approach which predicted the optimal required affinity of the hapten antibody binding step in PRIT, the MIT team, using directed evolution and yeast surface display was able to affinity mature by more than 1000-fold to low picomolar (pM), the original 2D12.5 antibody specific for benzyl(Bn)-DOTA-metal complex, now reformatted as the “C825” single-chain variable fragment (scFv) [16]. Next, the MIT team engineered BsAbs incorporating the C825 sequence utilizing a highly-modular IgG-scFv scaffold (e.g., anti-CEA/C825) [17]. These IgG-scFv antibody compositions are tetravalent molecules, consisting of two binding sites for tumor antigen (typically, about 10 nM), and two binding sites for the Bn-DOTA-radiometal hapten (~10–20 pM).
We recently adopted this methodology for PRIT by focusing on anti-GD2 antibodies with a documented record of safe use and proven tumor localization in humans [18]. For CRC, we reasoned that the GPA33 target could be ideal for PRIT. GPA33 is a transmembrane glycoprotein abundantly expressed in over 95% of CRCs, with restricted normal-tissue expression (colon and bowel epithelium) [19]. It exhibits long-term residence in cell membrane in tumors, relative to intestine, with minimal internalization and minimal vascular shedding. Using a variety of radiolabeled antibody forms, including initially, a murine monoclonal antibody (mAb) (A33 [20]) and subsequently a humanized version (huA33 [21]) this antigen is one of the most extensively studied targets in vivo. For example, we have performed 124I-huA33 imaging in CRC patients and confirmed through kinetic modeling that the differential clearance between Ag-positive tumor and Ag- positive intestine would yield TIs sufficient for tumor response by PRIT [8, 22, 23].
As we developed PRIT, the need for radiation dosimetry, based on quantitative imaging for measurement of time-dependent activity distribution in tumor and normal tissues, for optimal translation of PRIT to man became apparent. Combined therapeutic and diagnostic, or “theranostic,” PRIT refers to the use of reagents with virtually identical chemistries as vehicles for both diagnostic and therapeutic applications. We previously described successful applications of this approach using PET imaging of iodine-124 as a surrogate for iodine-131 therapy in thyroid cancer [24–26].
In the present studies, we applied lutetium-177 (177Lu) as both the therapeutic (β-emission for RIT) and diagnostic (γ-emission for imaging) radionuclide, and 177Lu itself, and position-emitting yttrium-86 (86Y) as its diagnostic surrogate. Furthermore, we emphasized 177Lu because the lower β-emission energy and short path length make it more likely to eradicate small tumors, in comparison to a longer path β-emission length radionuclide such as yttrium-90. In the current report, we test the principal hypothesis that optimized theranostic PRIT based on GPA33 antigen (Ag) tumor targeting and a BsAb-huA33 construct can be used to achieve the high TIs required for effective and safe CRC therapy in mouse xenografts and, ultimately, in man.
MATERIALS AND METHODS
Cloning and expression of huA33-C825
HuA33-C825 was prepared using the sequences for humanized A33 (huA33) [21] and C825 [16], a murine scFv antibody with high affinity for DOTA-radiometal complexes (Fig. 1a). The BsAb was made using the same platform as control hu3F8-C825 [18], only replacing variable regions (VH and VL) of hu3F8 with those of huA33 [21]. The IgG-scFv BsAb (molecular weight ~210-kDa) was produced in CHO cells and purified by protein A affinity chromatography as previously described [18].
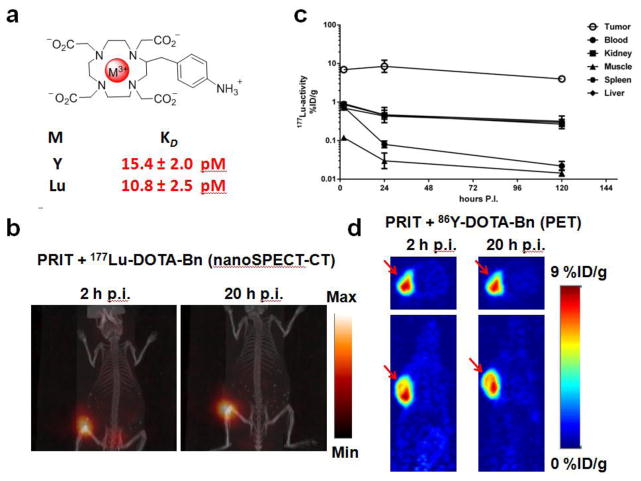
Fig 1.
(a) Structure of M-DOTA-Bn and C825 scFv affinities for either Y-DOTA-Bn or Lu-DOTA-Bn as reported by Orcutt, et al. [11]. (b) X-SPECT/CT maximum intensity projection images of a SW1222 tumor-bearing mouse following anti-GPA33 PRIT with 177Lu-DOTA-Bn. The following were administered for PRIT: 0.1 mg of BsAb, 40 μg of CA, and 7.4 MBq 177Lu-DOTA-Bn at 2 hr (left) and 20 hr (right) p.i. (note: same mouse is shown). (c) Decay-corrected 177Lu activity biodistribution curves for SW1222 tumor as well as select normal tissues from 2–120 h p.i. following huA33-C825 PRIT + 177Lu-DOTA-Bn (1.85–2.0 MBq; ~10 pmol). (d) Representative non-invasive serial PET images of nude mice bearing a s.c. SW1222 in the shoulder (red arrow) at 2 and 20 h p.i. (note: same mouse is shown) of huA33-C825 PRIT + 86Y-DOTA-Bn (8.6-8.8 MBq; ~50 pmol). Transverse and coronal slices through the tumor are shown. The images have been corrected for decay to the time of injection and calibrated for quantitation of activity in regions of interest and scaled to the same intensity.
Surface plasmon resonance studies
Biacore T100 Biosensor, CM5 sensor chip, and related reagents were purchased from GE Healthcare. The human GPA33 recombinant protein was purchased from Novoprotein. A BSA-(Y)-DOTA-Bn conjugate was prepared as previously described [18]. Both antigens were immobilized using the Amine Coupling Kit (GE Healthcare). Purified BsAbs and control antibodies were analyzed and data fit to a bivalent analyte model using the Biacore T100 evaluation software as previously described [18].
Anti-GPA33 PRIT reagents, protocol, and xenograft studies
The GPA33 (+) human CRC cell line SW1222 was obtained from the Ludwig Institute for Cancer Immunotherapy (New York, NY). SW1222 is notable among commonly investigated human CRC cell lines for preclinical RIT [27–30] in that it forms relatively well-differentiated and highly vascular subcutaneous (s.c.) tumors and is thus well-suited for antibody targeting [31]. 131I-A33 was used to demonstrate that 68 Gy to SW1222 tumors resulted in cures [29]. Details regarding the culturing of SW1222 and inoculation into immunocompromised mice are provided in Supplemental Information. Established tumors (50–700 mm3) were observed in 7–10 days; tumor volumes were estimated using the formula for the volume (V) of an ellipsoid (length/2×width/2×height/2). All reagents were administered intravenously (i.v.) via the lateral tail vein. The anti-GPA33 PRIT protocol included injections of three reagents; huA33-C825 BsAb (0.25 mg, 1.19 nmol) was injected first to allow localization in the tumor [t = −28 h]. Twenty four h later [t = −4 h] circulating BsAb was cleared using a CA (62.5 μg; 0.125 nmol; CA is a 500-kDa dextran-(Y)-DOTA-Bn conjugate, prepared according to Orcutt, et al. [32] and formulated in saline for injection; 7.625 nmol of (Y)-DOTA-Bn) and last, 4 h after CA the 177Lu-DOTA-Bn was injected [t = 0 h]. Radiolabeled DOTA-Bn was prepared as previously described [18] by incubating DOTA-Bn (p-NH2-Bn-DOTA; MW is 655 Da; Macrocyclics) and Lu-177 (carrier added; specific activity ~1110 GBq/mg; Perkin Elmer) or Y-86 (carrier free; specific activity ~1110 GBq/mg; Radiological Chemistry and Imaging Laboratory at Washington University in St. Louis) at 80°C for 1 h, and formulated in saline for injection. See Supplementary Information, Fig. 1 for illustration of PRIT strategy.
Micro single-photon emission computed tomography integrated with X-ray computed tomography (X-SPECT/CT) imaging was conducted on select mice during PRIT optimization. In addition, separate biodistribution studies were conducted with huA33-C825 trace radiolabeled with I-131 (Nordion) to estimate tumor uptake during PRIT (i.e., at 24 h post-injection (p.i.)). Details regarding X-SPECT/CT imaging, tracer preparation and quality control, as well as the ex vivo biodistribution analysis of radioactivity following radiotracer injection is described in Supplemental Information.
PET imaging of anti-GPA33 PRIT + 86Y-DOTA-Bn
A single group of mice bearing GPA33(+) SW1222 tumors in the shoulder (n = 5) were given PRIT (as described above) using 8.6–8.8 MBq (~50 pmol) of 86Y-DOTA-Bn, and non-invasively imaged at approximately 2 and 20 h p.i using a microPET Focus 120 (CTI Molecular Imaging, Inc. Knoxville, TN) and previously described methods [33] (see Supplemental Information for a detailed description of the imaging protocol).
Estimation of absorbed doses
Groups of GPA33 (+) SW1222 tumor-bearing mice (n = 4–5) were given PRIT + 1.85–2.0 MBq (~10 pmol) of 177Lu-DOTA-Bn and sacrificed at 2 (n = 5), 24 (n = 4), and 120 h p.i. (n = 5) for biodistribution analysis of 177Lu-activity in tumor and select normal tissues. Details regarding calculation of estimated absorbed doses are provided in Supplemental Information.
Titration of administered 177Lu-DOTA-Bn activity following PRIT with optimized BsAb and CA doses
To determine the effect of the 177Lu-DOTA-Bn dose on the relative uptake of 177Lu-DOTA-Bn in tumor and kidney during PRIT, groups of tumor-bearing mice (n = 5/group) were given PRIT + 11.1 (11.1–11.4; 60 pmol), 55.5 (54.6–55.1; 300 pmol), or 111 MBq (109.5–112.5 MBq; 600 pmol) of 177Lu-DOTA-Bn. All groups were sacrificed at 24 h p.i. of 177Lu-DOTA-Bn for biodistribution analysis of 177Lu activity. These data were plotted in combination with aforementioned PRIT + 1.85–2.0 MBq (~10 pmol) biodistribution data of 177Lu-activity in tumor and kidney to estimate tissue saturation of 177Lu-DOTA-Bn during PRIT. In addition to the quantification of activity by gamma counting, the kidneys were collected from animals given PRIT + 11.1–111 MBq and frozen at −80 oC in OCT for autoradiography and histochemistry analysis (see Supplemental Information for a detailed description of the autoradiography and histochemistry protocol).
Theranostic 177Lu-DOTA-Bn treatment using optimized PRIT strategy
Groups of SW1222 tumor-bearing mice (50–150 mm3) were injected with either huA33-C825 or non-specific (n.s.) IgG-C825 PRIT (i.e., single-cycle treatment, BsAb injection on day 6, CA/177Lu-DOTA-Bn injections on day 7 post-tumor inoculation) or two cycles of PRIT (i.e., fractionated treatment, BsAb injections on days 9 and 16 and CA/177Lu-DOTA-Bn injections given on day 10 and day 17 post-tumor inoculation). For PRIT with n.s. IgG-C825, an equivalent mg dose of the GD2-targeted BsAb hu3F8-C825 [18] was used in place of huA33-C825, since it does not cross react with SW1222 tumor. Tumor volumes were measured three times a week and the following definitions were used to describe treatment response: a complete response (CR) is defined as tumor shrinkage to 20% of initial tumor volume during treatment; a partial response (PR) is defined as a tumor showing no change in growth between successive measurements or any other tumor shrinkage not considered at CR; excessive tumor burden is defined as a final volume of >2000 mm3. Non-invasive planar scintigraphy of treated mice was conducted at 20 h p.i. of 177Lu-DOTA-Bn (see Supplemental Information for imaging method details) to verify tumor-specific versus non- specific targeting as well as assess whole-body clearance of mice receiving 177Lu-DOTA-Bn alone [34]. Animals were observed until they required sacrifice due to excessive tumor burden (unless otherwise noted). If a weight loss greater than 15% of their initial body weight in one or two days, or 20% or more of the mouse’s starting weight was observed, then the animal was removed from the group at that time and sacrificed. To further evaluate toxicity, a total of eight randomly selected animals undergoing treatment were submitted for histopathologic assessment of the site of s.c. tumor, kidney, bone marrow (sternum, vertebrae, femur, and tibia), liver, and spleen by board-certified pathologists at the Memorial Sloan Kettering Cancer Center Laboratory of Comparative Pathology. Also from these mice, immunohistochemical (IHC) staining for the presence of GPA33 of was carried out using previously described protocols [8]. The maximum tolerated dose (MTD) was not determined during the current study.
RESULTS
In vitro characterization of huA33-C825
Biochemical purity analysis of huA33-C825 by SE-HPLC is shown in Supplementary, Information Fig. 2a. SE-HPLC showed a major peak (90% by UV analysis) with an approximate MW of 210-kDa, as well as some minor peaks assumed to be aggregates removable by gel filtration. The BsAb remained stable by size-exclusion high-pressure liquid chromatography and Biacore after multiple freeze and thaw cycles (data not shown). The binding affinity was measured by Biacore T100. For GPA33, huA33-C825 had a kon of 9.15x104 M−1s−1, a koff of 5.81x10−3 s−1, and overall KD of 63.5 nM–less than the parental huA33-IgG1 (kon of 6.14x105 M−1s−1, koff of 1.05x10−3s−1, and overall KD of 1.71 nM) (Supplementary Information, Fig. 2b). For antigen BSA-(Y)-DOTA-Bn, huA33-C825 had a kon of 1.90x104 M−1s−1, a koff of 2.20x10−4 s−1, and overall KD of 11.6 nM–comparable to control IgG-C825 (the anti-GD2 BsAb hu3F8-C825) (kon of 1.60x104 M−1s−1, koff of 3.37x10−4 s−1, and overall KD of 21.2 nM) (Supplementary Information, Fig. 2c). In summary, huA33-C825 retained high binding affinity to BSA-(Y)-DOTA-Bn, but lost considerable affinity to antigen GPA33.
Optimization of PRIT with huA33-C825, CA, and 177Lu-DOTA-Bn
A combination of X-SPECT/CT imaging and ex vivo biodistribution studies of huA33-C825 PRIT + 177Lu-DOTA-Bn were used to optimize huA33-C825 and CA doses during PRIT with the aims of achieving high uptake at the tumor while simultaneously minimizing exposure to radiosensitive normal organs such as the blood and kidney (see Supplemental Information, Fig. 3). A representative X-SPECT/CT image following PRIT + 177Lu-DOTA-Bn targeting of GPA33 (+) to a SW1222 xenograft in vivo is shown in Fig. 1b. The 131I-huA33-C825 uptake in tumor at 24 h p.i. (average ± standard deviation (SD)) was 3.71 ± 1.00 %ID/g (see Supplemental Information, Table 2 for tabulated activity values). Taking into account the tracer-specific activity and dose, this corresponds to an absolute huA33-C825 uptake of 44 pmol/g (with ~50% IR, then 88 pmol/g).
Absorbed dose estimates for optimized PRIT + 177Lu-DOTA-Bn
Decay-corrected time-activity curves up to 120 h p.i. of 177Lu-DOTA-Bn for tumor, kidneys, liver, spleen, and blood are shown in Fig. 1c (see Supplemental Information, Table 1 for activity values). The estimated absorbed doses of 177Lu-DOTA-Bn (as cGy/MBq) for blood, tumor, liver, spleen, and kidneys were 0.9, 65.8, 6.3, 6.6, and 5.3, respectively (Table 1). The ratio of absorbed dose estimates for tumor to those for select normal tissues (i.e., TI) ranged from ~10 (e.g., for liver, spleen, and kidney) to ~220 for muscle (Table 1).
Table 1.
Absorbed doses for anti-GPA33 PRIT with 177Lu-DOTA-Bn in nude mice carrying s.c. GPA33(+)-SW1222 tumors.
| Tissues | cGy/MBq | Therapeutic Index |
|---|---|---|
| Blood | 0.9 | 73 |
| Tumor | 65.8 | |
| Heart | 1.4 | 47 |
| Lung | 1.8 | 37 |
| Liver | 6.3 | 10 |
| Spleen | 6.6 | 10 |
| Stomach | 0.6 | 110 |
| Small | 0.5 | 132 |
| Intestine | ||
| Large | 0.8 | 82 |
| Intestine | ||
| Kidneys | 5.3 | 12 |
| Muscle | 0.3 | 219 |
| Bone | 0.6 | 110 |
Serial non-invasive PET imaging of GPA33 (+) xenografts with PRIT + 86Y-DOTA-Bn
Representative PET images of SW1222 tumor-bearing mice at 2 and 20 h p.i. of PRIT + 86Y-DOTA-Bn are shown in Fig. 1d. The corresponding maximum intensity projection PET images are provided in Supplemental Information, Fig. 4. The CRC xenograft in the shoulder can be clearly delineated with minimal uptake in normal tissue and thus high overall contrast. The uptake in tumor was quantified by region-of-interest analysis of each of the calibrated PET images, and was determined to be 8.75 ± 0.91 and 8.44 ± 1.01 %ID/g (average ± standard error of the mean (SEM); n = 5) at 2 and 20 h p.i., respectively, indicating rapid uptake of 86Y-DOTA-Bn (presumably by tumor-associated huA33-C825) and slow subsequent clearance of the huA33-C825/86Y-DOTA-Bn complex from the tumor. The tumor uptake determined by ex vivo biodistribution at 24 h p.i. was 7.85 ± 0.65 %ID/g (average ± SEM). A comparison of the tumor uptake determined by either PET imaging or biodistribution of PRIT with either 86Y-DOTA-Bn or 177Lu-DOTA-Bn at 2 and 24 h p.i. is shown in Supplemental Information, Fig. 5. No significant difference (P > 0.05) was observed between the two isotopes at either time point, suggesting good correlation between the in vivo fates of the two distinct M-DOTA-Bn haptens following PRIT.
Titration of 177Lu-DOTA-Bn administered activity during PRIT
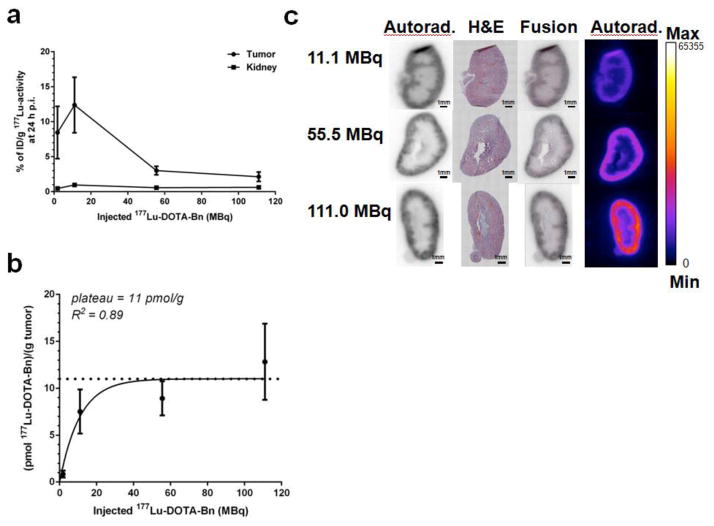
The uptake in tumor and kidney at 24 h p.i. (as %ID/g, average ± SD) as a function of administered 177Lu-DOTA-Bn activity from ~2-111 MBq is shown in Fig. 2a. While the relative 177Lu activity decreased ~4-fold for tumor with increasing administered activity (e.g., ~8.5 to ~2% ID/g for 2 MBq and 111 MBq, respectively), the relative 177Lu activity in kidney remained <1% ID/g and relatively constant (average range: 0.46–0.96 %ID/g), suggesting that saturation of available specific M-DOTA-Bn receptors by hapten were occurring in the tumor but not in the kidney compartments. The absolute 177Lu activity uptake in tumor (as pmol/g of tumor) is shown in Fig. 2b, and was fitted to a one-phase association equation (GraphPad, Prism version 6.00), with saturation apparent at a 177Lu-DOTA-Bn activity of ~11 pmol/g of tumor; R2 = 0.89. Thus during PRIT therapy studies, 177Lu-DOTA-Bn activities 55 MBq were considered subsaturating. Representative autoradiography and histochemistry data for kidney for three different 177Lu-DOTA-Bn activity levels (11.1, 55.5, and 111.0 MBq) is shown in Fig. 2c. The 177Lu-activity appears to be distributed predominantly in the renal cortex, with higher cortex-to-medulla activity concentration ratios with increasing administered 177Lu-DOTA-Bn activity.
Fig 2.
(a) 177Lu activities in tumor and kidney determined by ex vivo biodistribution (as %ID/g) for anti-GPA33 PRIT + 177Lu-DOTA-Bn doses ranging from ~2–111 MBq. (b) Absolute 177Lu activity in tumor (as pmol of 177Lu per gram of tumor) for anti-GPA33 PRIT + 177Lu-DOTA-Bn doses ranging from ~2–111 MBq. (c) Representative ex vivo autoradiograms (autorad.) and IHC (H&E) images of kidney following anti-GPA33 PRIT + three different 177Lu-DOTA-Bn administered activities at 24 h p.i. (11.1, 55.5, and 111.0 MBq, respectively).
PRIT Therapy Studies
A total of three separate therapy studies with 177Lu-DOTA-Bn were conducted in SW1222 tumor-bearing mice to evaluate efficacy. A summary of all tumor response data as well as estimated absorbed doses for tumor, blood, and kidney for all PRIT treatment groups is provided in Table 2. No significant weight loss was observed in any of the control or treatment groups (data not shown).
Table 2.
Anti-GPA33 PRIT treatment studies with either single or dual-cycle anti-GPA33 PRIT + 177Lu-DOTA-Bn administered activities up to 111 MBq.
| Anti- GPA33 PRIT treatment | 177Lu-DOTA-Bn administered activity | Tumor absorbed dose (cGy)* | Blood absorbed dose (cGy)** | Kidney absorbed dose (cGy)*** | Complete Response | Survival at 140 d | Time to tumor recurrence 500 mm2 |
|---|---|---|---|---|---|---|---|
| Single-cycle | |||||||
| 11.1 MBq | 730 | 10 | 59 | 0/8 | 0/8 | ||
| 33.3 MBq | 2191 | 30 | 176 | 0/8 | 0/8 | ||
| 111 MBq | 2580a | 100 | 588 | 2/5 | 1/5 | 65 d | |
| Dual-cycle | |||||||
| 2 x 11.1 MBq (22.2 MBq) | 1460 | 20 | 118 | 2/5 | 0/5 | 9, 36 d median: 22.5 |
|
| 2 x 33.3 MBq (66.6 MBq) | 4382 | 60 | 354 | 5/5 | 1/5 | 7, 12, 23, 65 d median: 17.5 |
|
| 2 x 55.5 MBq (111.0 MBq) | 7304 | 100 | 588 | 4/4 | 1/4 | 34, 42, 45 d median: 42 |
65.8 cGy/MBq based on average tumor uptake of 8.5% ID/g at 24 h p.i.
0.9 cGy/MBq
5.3 cGy/MBq
adjusted for average tumor uptake of 3% ID/g at 24 h p.i. to account for saturating dose
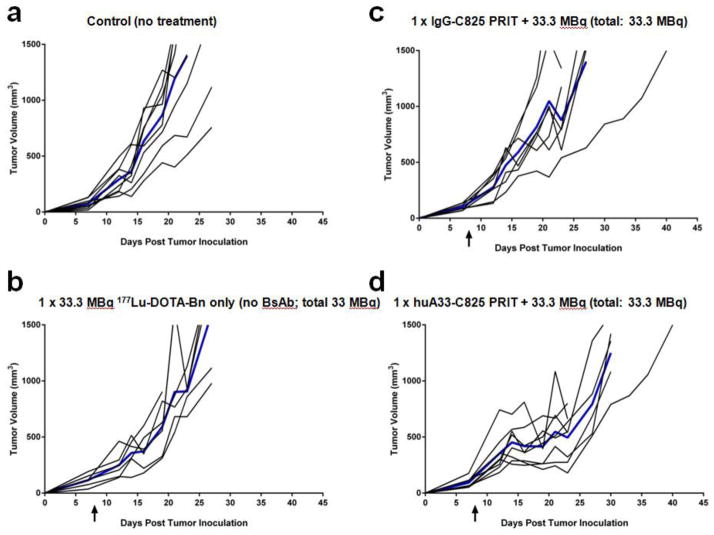
First, 5 groups of tumor-bearing mice (n = 6–8/group; 7-day old tumors, tumor volumes (TV): 100–200 mm3) were treated with either: vehicle alone (i.e., untreated, n = 8), 33.3 MBq 177Lu-DOTA-Bn alone (vehicle given during BsAb and CA injections, n = 6), n.s. hu3F8-C825 PRIT + 33.3 MBq 177Lu-DOTA-Bn (control antibody, n = 8), or huA33-C825 PRIT + either 11.1 MBq or 33.3 MBq 177Lu-DOTA-Bn (antibody of interest, both n = 8). Graphs depicting tumor response data from treatment groups are shown in Fig. 3 and Supplemental Information, Fig.6. Representative scintigraphy images of select treated mice are shown in Supplemental Information, Fig. 7 and verify specific tumor targeting during PRIT including huA33-C825 and 177Lu-DOTA-Bn compared with controls and n.s. PRIT. To summarize efficacy, no CRs were produced from any of the GPA33-targeted PRIT treatment or control groups. Also, unremarkable PRs producing slight tumor growth delays were observed only in groups treated with huA33-C825 PRIT + administered activity up to 33.3 MBq 177Lu-DOTA-Bn.
Fig 3.
Tumor response data for therapy studies including: (a) control (no treatment), (b) single-cycle treatment with 177Lu-DOTA-Bn only (i.e., no BsAb or CA; 177Lu-DOTA-Bn administered activity 33.3 MBq), (c) n.s. hu3F8-C825 PRIT (177Lu-DOTA-Bn administered activity: 33.3 MBq), and (d) anti-GPA33 PRIT (177Lu-DOTA-Bn administered activity: 33.3 MBq).
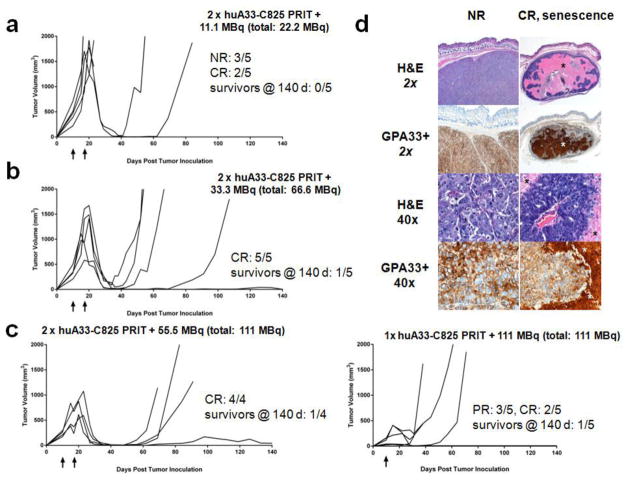
In the second therapy study, a two-cycle regimen of huA33-C825 PRIT treatment was investigated (n = 4–5/group; 10-day old tumors, TV10: 200–700 mm3). Similar to the first treatment study, untreated mice (n = 5) required sacrifice within 30 days due to excessive tumor burden, and the time to reach 500 mm3 was 13 ± 2 d (data not shown). For treatment with 2 x huA33-C825 PRIT + 11.1 MBq 177Lu-DOTA-Bn (total 177Lu-DOTA-Bn administered activity 22.2 MBq; estimated tumor absorbed dose 15 Gy) (n = 5), 2/5 animals showed CR (Fig. 4a). In the recurrent tumors, the time to reach 500 mm3 was 9 d or 36 d. Treatment with 2 x huA33-C825 PRIT + 33.3 MBq 177Lu-DOTA-Bn (total 177Lu-DOTA-Bn administered activity 66.6 MBq; estimated tumor absorbed dose 44 Gy) (n = 5) produced 5/5 CR (Fig. 4b). In the recurrent tumors, the time to reach 500 mm3 was 7 d, 12 d, 23 d, and 65 d, and a single mouse showed a tumor size of <10 mm3 at 140 d (average time to recurrent tumor of 500 mm3 was 27 ± 26 d). Treatment with two cycles of huA33-C825 PRIT + 55.5 MBq 177Lu-DOTA-Bn (total 177Lu-DOTA-Bn administered activity 111.0 MBq estimated tumor absorbed dose 73 Gy) (n = 4) produced 4/4 CR (Fig. 4c, left). In the recurrent tumors, the time to reach 500 mm3 was 34 d, 42 d, and 45 d, and a single mouse had a tumor size of 44 mm3 at 140 d (average time to recurrent tumor of 500 mm3 was 40 ± 6 d). No major treatment-related toxicities were observed in 8/8 mice randomly selected for necropsy and pathologic assessment (pathologist summary provided in Supplemental Information). In addition, IHC staining of the tumor sections for the presence of GPA33 revealed that the residual tumor was GPA33-positive (Fig. 4d).
Fig 4.
Tumor response data for dual-cycle PRIT + (a) 11.1 MBq 177Lu-DOTA-Bn (total: 22.2 MBq), + (b) 33.3 MBq 177Lu-DOTA-Bn (total: 66.6 MBq) or + (c, left) 55.5 MBq177Lu-DOTA-Bn (total: 111 MBq). Tumor response data for single-cycle PRIT + 111 MBq of 177Lu-DOTA-Bn is also provided (c, right). (d) Morphologic assessment and IHC detection of GPA33 antigen of select formalin-fixed, paraffin-embedded tumors from GPA33-PRIT treated mice. Left panel: A non-responding (NR) tumor (tumor size at sacrifice: >1500 mm3) following treatment with 2 x huA33-C825 PRIT + 11.1 MBq (total: 22.2 MBq). Right panel: A CR tumor showing senescence at 140 d, treated with 2 x huA33-C825 PRIT + 55.5 MBq (total: 111 MBq). Five-μm-thick sections were stained with hematoxylin and eosin (H&E) and immunohistochemically analyzed with murine monoclonal antibody A33 for presence of GPA33 antigen (GPA33+). The NR tumor appears as a bulky mass displaying little to no necrosis in the solid antigen-positive tumor areas. High magnification shows intact, large tumor cells without necrotic changes. The CR senescent tumor is considerably smaller in size (~40 mm3 when sacrificed at 140 d) compared to the NR tumor displaying large necrotic areas (*) in the center with surrounding partially papillary-shaped vital tumor formations. High-magnification regions show a papillary tumor area with central stroma core and surrounding GPA33-positive necrotic areas.
Following these PRIT treatment studies, we made some very approximate estimates of the number of clonogenic tumor cells remaining after dual-cycle treatment with huA33-C825 PRIT + 55 MBq 177Lu-DOTA-Bn per mouse. We noted that the individual tumors that responded to treatment with a CR or greater dropped below the detection (palpation) threshold at about 12 days after the second treatment dose, or at about 30 days after inoculation of tumor cells (Fig. 4c). This was used as time zero, from which regrowth of clonogenic cells would begin to a volume of 500 mm3 (i.e., recurrence). The estimated tumor absorbed dose of 73 Gy resulted in CRs in 4/4 mice including a single survivor without recurrence at 140 d. Based on an observed tumor doubling time of 5 d, the 3 tumors that recurred at approximately 35, 40, and 45 d (7–9 doublings) after the nadir had an implied cell kill of 2–3 logs. For the tumor without visible re-growth during the 140 d observation period, it is more difficult to estimate a cell kill because it may or may not have been cured. If the tumor had recurred at 140 d (28 doublings) post nadir, the implied cell kill would have been approximately 8 logs. If we assume some numerical value for clonogenic cell density, it is possible to calculate how many logs of cell kill would be required to produce a significant probability of cure. For example, a clonogenic cell density of 109 cells per gram would imply that approximately 9 logs of kill are required to reduce a 500 mg tumor to a single surviving clonogenic cell. However, if the true number was 106 cells per gram, then only 6 logs of kill would be required. These values (and their associated uncertainties) translate into the amount of radiation dose that would be required to achieve a significant level of tumor cure. If an estimated tumor dose of 73 Gy gives approximately 3 logs of kill but 9 logs are needed (i.e., 109 cells/g), then the dose would have to be three times greater (i.e., 220 Gy). In contrast, if only 6 logs are needed (i.e., 106 cells/g) then the dose would need to be two times greater (i.e., 150 Gy). However, if the true number of clonogenic cells per gram was only 103 (e.g., a cancer stem cell-like scenario), doses similar to the current estimated value of 73 Gy would already be sufficiently high to produce a significant likelihood of tumor cure. Our experimental observation of a 25% probability of cure seems to most closely resemble the last of these scenarios. Of course, in reality, we do not know what the clonogenic tumor cell density is and tumor regression and regrowth is likely to be more complex than a simple monoexponential function, so these speculative calculations should be considered with due caution.
Finally, a third PRIT therapy study was performed at a 177Lu-DOTA-Bn dose of 111 MBq, administered as a single cycle to compare treatment with single versus fractionated PRIT dosing (n= 5/group; 10-day old tumors, TV10: 50–300 mm3). All untreated mice showed similar tumor growth kinetics to previous studies (tumor doubling time: ~5 d, data not shown). PRIT treatment produced 3/5 PR as well as 2/5 CR with delayed recurrence in 1/5 mice (the time to reach 500 mm3 was 65 d) and a single survivor without recurrence at 140 d (at 140 d post-tumor inoculation no measurable tumor at the site of s.c. injection; TV10: 121 mm3) (Fig. 4c, right). A single treated mouse showing a PR required sacrifice due to (non-treatment related) mobility concerns 18 days post-treatment.
DISCUSSION
We achieved our goal of demonstrating that a dosimetry-based TI could be used as a bench mark for successful therapeutic targeting by PRIT in CRC. We demonstrated mean TIs of ~73 for blood and 12 for kidney, and an estimated tumor dose of 43–73 Gy with an estimated 2.1–3.0 log kill at a 73-Gy tumor absorbed dose, without detectable bone marrow and renal toxicity. We employed 177Lu-DOTA-Bn for theranostic RIT with administered activities up to 111 MBq delivered as a fractionated dose strategy (e.g., 2 x huA33-C825 PRIT + 55.5 MBq) to produce CR with 100% frequency, including survival beyond 140 d in 2/9 mice, with no major treatment-related toxicities observed. IHC analysis of the residual tumors revealed GPA33 (+) phenotype, suggesting that the reason for recurrence of some tumors and was due to insufficient tumor absorbed dose rather than by escape by genetic mutation or by some other mechanism. Also, we documented that PRIT is a highly flexible approach that is readily adaptable to multiple dosing regimens, and in fact divided doses were more effective than equivalent single doses in inducing CRs. Based on the absence of any treatment-relate toxicities, all of our therapeutic regimes delivered normal-tissue absorbed doses well below the respective normal-tissue MTDs. Dose escalation could therefore safely proceed in this setting.
At MTD, we anticipate the normal tissues to be most sensitive to off-target radiotoxicity during anti-GPA33 PRIT to be bone marrow (using blood as a dosimetric surrogate for bone marrow), lung, liver, and kidneys (estimated absorbed doses: 0.9, 1.8, 6.3, and 5.3 cGy/MBq, respectively; Table 1). Based on human normal-tissue radiation dose tolerance estimates derived from clinical observations [35], the maximum tolerated doses are 250 cGy for bone marrow, 1500 cGy for lung, 3000 cGy for liver, and 2000 cGy for kidney. Therefore we estimate the maximum tolerated pretargeted 177Lu-DOTA-Bn activity to be 278 MBq, with the bone marrow as the dose limiting-organ. At this activity, the estimated absorbed dose delivered to tumor would be 18292 cGy (183 Gy), with 250 cGy to blood (marrow) and 1473 cGy to kidney.
We demonstrated that serial non-invasive X-SPECT/CT imaging of s.c. GPA33 (+) tumors was possible with PRIT with 177Lu-DOTA-Bn, suggesting quantitative imaging combined with therapy is possible (e.g. with QSPECT [36]). In addition, we demonstrated that serial non-invasive PET imaging of s.c. GPA33 (+) tumors was possible with PRIT with 86Y-DOTA-Bn. It should be noted that the affinities for C825 binding of yttrium and lutetium are virtually identical and in the picomolar range. 177Lu has a considerably longer half-life than 86Y (6.73 days (d) versus 14.7 hours (h), respectively). Even so, the kinetics of rapid localization and long-term retention of the M-DOTA-Bn favors the use of 86Y because the half-life is long enough to accurately determine in tumor the time course of uptake, the maximum uptake, and the subsequent rate of clearance of the plateau phase of slow clearance of the targeted therapeutic radiolanthanide. Furthermore, the half-life of 86Y is sufficiently short to not appreciably increase the absorbed doses to normal organs during 177Lu-based PRIT therapy and could also allow repeated measurements before each cycle of a fractionated approach. Thus, 86Y is a suitable surrogate diagnostic for 177Lu. Further, PET imaging is more quantitatively accurate than other nuclear imaging methods such as single-photon emission computed tomography (SPECT) with 177Lu and in general. Finally, 86Y could be used as a pre-therapy tracer for RIT dosimetry with either 90Y or 177Lu during anti-GPA33 PRIT. Yttrium-86 has a complex spectrum, and radiation exposure is not insignificant. However, in a theranostic application, where much larger treatment doses are anticipated, the gain of use will outweigh the risk, because of the potential for improving patient selection and treatment planning. Also, corrections must be made for optimal reconstruction; notably, in addition to positrons, the large number of prompt gamma rays emitted by 86Y can interfere with PET image quantitation, but accuracy can be significantly improved with correction algorithms [32, 33].
During our investigation of anti-GPA33 PRIT, we made some notable observations regarding opportunities for improvement of current targeting reagents. First, the binding affinity of huA33 for its cognate antigen was reduced when the C825 was attached. Although we were able to achieve high TIs and apparently prolonged tumor retention during in vivo PRIT, the in vitro binding affinity data (Biacore) showed that the huA33-C825 lost considerable affinity for antigen GPA33 as compared to parental huA33-IgG. This is consistent with modeling predictions of the affinity dependence of tumor uptake, which indicate that large molecules (e.g., IgGs) are able to achieve similar uptake levels at much lower affinities in the 10−8 to 10−6 mol/L Kd range, presumably due to the slow rate of intravasation allowing for repeated binding within the tumor [34, 35]. The C825 affinity for radiolanthanide Bn DOTA was unaffected. Nonetheless, we do not rule out that tumor targeting could be improved by affinity recovery, as well as by addressing other issues related to the pharmacokinetics and biodistribution of the BsAb [36]. These issues are currently under study. Second, uptake of the M-DOTA-Bn by antibody prelocalized to tumor was only 10% of that predicted, on a picomolar basis. We speculate that the low maximum capacity is likely due to a combination of factors including the metabolism of the dextran-CA and subsequent leakage of small DOTA-dextran fragments into circulation, and the in vivo stability (e.g., proteolytic) of the DOTA-binding scFv C825. Future studies will include alternative CA with improved serum stability, to reduce the likelihood of small DOTA-hapten into circulation following hepatic metabolism as well as using no-carrier-added formulations of 177Lu-DOTA-Bn, which could offer an ~3-fold improvement of 177Lu-specific activity (from ~1,110 GBq/mg to a theoretical specific activity of 4,033 GBq/mg Lu).
Successful development of a theranostic PRIT approach in CRC would meet an unmet need for cancer detection and management, and a platform-based PRIT treatment paradigm may indeed be feasible using a variety of IgG-C825 antibodies targeting human cancers. Our previous investigations of PRIT, including PRIT of neuroblastoma based on hu3F8-C825 [18], supports the practicality of the platform concept. For one thing, once optimal clearing reagents and C825-haptens have been developed, these same reagents can be used as a component of theranostic approaches in combination with bifunctional antibody forms of Ag specificities for a broad spectrum of human tumors. In addition, direct applicability to most human tumors provides several possible advantages: First, radiation effects at high absorbed dose have totally distinct pattern of resistance from that of current drugs and (non-radioactive) antibodies, and we therefore expect complementary effects to current drugs. Second, utilizing a theranostic approach, both diagnostic and therapeutic selection information can be obtained at “tracer doses.” This permits optimized patient dosing and avoidance of treating patients whose tumors are not targeted by the antibody and therefore who are not likely to benefit from this treatment because of low tumor radiation dose or high dose to critical organs such as kidney and gut. Furthermore, in this study we characterized the importance of mass used in the 3 targeting steps and their effect on tumor localization and normal organ uptake. Similar data will need to be obtained in patients. To optimize these dose seeking studies a modeling approach may be utilized [42]. Finally, a modular therapy in which the radioactivity is separated from the antibody infusion is appealing in a clinical setting, leading to an inherently multi-disciplinary approach. For example, patient management and radiation safety measures are simplified.
CONCLUSIONS
Using a three-step PRIT protocol with the anti-GPA33/anti-DOTA(metal) BsAb huA33-C825, a dextran-based CA, and 177Lu-DOTA-Bn, mice with established s.c. SW1222 human CRC xenografts were treated with 177Lu-DOTA-Bn administered activities as high as 111 MBq without demonstrable radiotoxicity to normal tissues including bone marrow and kidney. Fractionated administered activities were significantly more effective than equivalent single activities in terms of tumor response. In addition, serial non-invasive PET imaging with pretargeted 86Y-DOTA-Bn was successfully demonstrated as a basis for optimizing diagnosis and for dosimetry of pretargeted 177Lu-DOTA-Bn and 86Y-DOTA-Bn for PRIT in a murine model of human CRC.
Supplementary Material
Acknowledgments
The authors would like to thank Donald Axworthy, Dr. Kelly Orcutt, and Dr. James Russell for helpful discussions. We gratefully acknowledge Teja Muralidhar Kalidindi, Valerie Longo, and the Memorial Sloan Kettering Small Animal Imaging Core Facility for support with experiments. The authors also thank Leah Bassity for her editorial work on the manuscript.
Funding: This study was supported in part by the following: Donna & Benjamin M. Rosen Chair (to SM Larson), Enid A. Haupt Chair (to NK Cheung), The Center for Targeted Radioimmunotherapy and Theranostics, Ludwig Center for Cancer Immunotherapy, Memorial Sloan Kettering Cancer Center (MSKCC) (to S.M. Larson), a training grant from the National Institutes of Health (R25-CA096945; PI: H. Hricak, fellow: S.M. Cheal), and a National Institutes of Health grant (R01-CA-101830; to K.D. Wittrup). S. M. Larson was also supported in part by P50-CA86438. Technical services provided by the Memorial Sloan Kettering Small-Animal Imaging Core Facility were supported by National Institutes of Health Grants R24-CA83084 (to H. Hricak), P30-CA08748 (to C. Thompson), and P50-CA92629 (to H. Scher). National Institutes of Health Shared Instrumentation Grant No 1 S10 RR028889-01 (to P.B. Zanzonico), and a Shared Resources Grant from the MSKCC Metastasis Research Center (to P. B. Zanzonico), which provided funding support for the purchase of the Focus 120 microPET and the NanoSPECT/CT Plus, respectively, are gratefully acknowledged.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Statement on the welfare of animals: All animal experiments were approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center, and institutional guidelines for the proper and humane use of animals in research were followed.
References
- 1.American Cancer Society. Colorectal Cancer Facts & Figures 2014–2016. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15:347–60. doi: 10.1038/nrc3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajjar G, Sharkey RM, Burton J, Zhang CH, Yeldell D, Matthies A, et al. Phase I radioimmunotherapy trial with iodine-131-labeled humanized MN-14 anti-carcinoembryonic antigen monoclonal antibody in patients with metastatic gastrointestinal and colorectal cancer. Clin Colorectal Cancer. 2002;2:31–42. doi: 10.3816/CCC.2002.n.009. [DOI] [PubMed] [Google Scholar]
- 4.Wong JYC, Chu DZ, Yamauchi DM, Williams LE, Liu A, Wilczynski S, et al. A phase I radioimmunotherapy trial evaluating 90yttrium-labeled anti-carcinoembryonic antigen (CEA) chimeric T84.66 in patients with metastatic CEA-producing malignancies. Clin Cancer Res. 2000;6:3855–63. [PubMed] [Google Scholar]
- 5.Goldenberg DM, Chang CH, Rossi EA, JW, McBride, Sharkey RM. Pretargeted molecular imaging and radioimmunotherapy. Theranostics. 2012;2:523–40. doi: 10.7150/thno.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagan PL, Halpern SE, Dillman RO, Shawler DL, Johnson DE, Chen A, et al. Tumor size: effect on monoclonal antibody uptake in tumor models. J Nucl Med. 1986;27:422–7. [PubMed] [Google Scholar]
- 7.Mayer A, Tsiompanou E, Flynn AA, Pedley RB, Dearling J, Boden R, et al. Higher dose and dose-rate in smaller tumors result in improved tumor control. Cancer Invest. 2003;21:382–8. doi: 10.1081/cnv-120018229. [DOI] [PubMed] [Google Scholar]
- 8.O'Donoghue JA, Smith-Jones PM, Humm JL, Ruan S, Pryma DA, Jungbluth AA, et al. 124I-huA33 antibody uptake is driven by A33 antigen concentration in tissues from colorectal cancer patients imaged by immuno-PET. J Nucl Med. 2011;52:1878–85. doi: 10.2967/jnumed.111.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stillebroer AB, Zegers CM, Boerman OC, Oosterwijk E, Mulders PF, O'Donoghue JA, et al. Dosimetric analysis of 177Lu-cG250 radioimmunotherapy in renal cell carcinoma patients: correlation with myelotoxicity and pretherapeutic absorbed dose predictions based on 111In-cG250 imaging. J Nucl Med. 2012;53:82–9. doi: 10.2967/jnumed.111.094896. [DOI] [PubMed] [Google Scholar]
- 10.O'Donoghue JA, Bardies M, Wheldon TE. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J Nucl Med. 1995;36:1902–9. [PubMed] [Google Scholar]
- 11.Reardan DT, Meares CF, Goodwin DA, McTigue M, David GS, Stone MR, et al. Antibodies against metal chelates. Nature. 1985;316:265–8. doi: 10.1038/316265a0. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin DA, Meares CF, Watanabe N, McTigue M, Chaovapong W, Ransone CM, et al. Pharmacokinetics of pretargeted monoclonal antibody 2D12.5 and 88Y-Janus-2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) in BALB/c mice with KHJJ mouse adenocarcinoma: a model for 90Y radioimmunotherapy. Cancer Res. 1994;54:5937–46. [PubMed] [Google Scholar]
- 13.Feng X, Pak RH, Kroger LA, Moran JK, DeNardo DG, Meares CF, et al. New anti-Cu-TETA and anti-Y-DOTA monoclonal antibodies for potential use in the pre-targeted delivery of radiopharmaceuticals to tumor. Hybridoma. 1998;17:125–32. doi: 10.1089/hyb.1998.17.125. [DOI] [PubMed] [Google Scholar]
- 14.Corneillie TM, Whetstone PA, Meares CF. Irreversibly binding anti-metal chelate antibodies: artificial receptors for pretargeting. J Inorg Biochem. 2006;100:882–90. doi: 10.1016/j.jinorgbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Chmura AJ, Orton MS, Meares CF. Antibodies with infinite affinity. P Natl Acad Sci USA. 2001;98:8480–4. doi: 10.1073/pnas.151260298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orcutt KD, Slusarczyk AL, Cieslewicz M, Ruiz-Yi B, Bhushan KR, Frangioni JV, et al. Engineering an antibody with picomolar affinity to DOTA chelates of multiple radionuclides for pretargeted radioimmunotherapy and imaging. Nucl Med Biol. 2011;38:223–33. doi: 10.1016/j.nucmedbio.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orcutt KD, Ackerman ME, Cieslewicz M, Quiroz E, Slusarczyk AL, Frangioni JV, et al. A modular IgG-scFv bispecific antibody topology. Protein Eng Des Sel. 2010;23:221–8. doi: 10.1093/protein/gzp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheal SM, Xu H, Guo HF, Zanzonico PB, Larson SM, Cheung NK. Preclinical evaluation of multistep targeting of diasialoganglioside GD2 using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex. Mol Cancer Ther. 2014;13:1803–12. doi: 10.1158/1535-7163.MCT-13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garinchesa P, Sakamoto J, Welt S, Real F, Rettig W, Old L. Organ-specific expression of the colon cancer antigen A33, a cell surface target for antibody-based therapy. Int J Oncol. 1996;9:465–71. doi: 10.3892/ijo.9.3.465. [DOI] [PubMed] [Google Scholar]
- 20.Welt S, Divgi CR, Kemeny N, Finn RD, Scott AM, Graham M, et al. Phase I/II study of iodine 131-labeled monoclonal antibody A33 in patients with advanced colon cancer. J Clin Oncol. 1994;12:1561–71. doi: 10.1200/JCO.1994.12.8.1561. [DOI] [PubMed] [Google Scholar]
- 21.King DJ, Antoniw P, Owens RJ, Adair JR, Haines AM, Farnsworth AP, et al. Preparation and preclinical evaluation of humanised A33 immunoconjugates for radioimmunotherapy. Br J Cancer. 1995;72:1364–72. doi: 10.1038/bjc.1995.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrasquillo JA, Pandit-Taskar N, O'Donoghue JA, Humm JL, Zanzonico P, Smith-Jones PM, et al. (124)I-huA33 antibody PET of colorectal cancer. J Nucl Med. 2011;52:1173–80. doi: 10.2967/jnumed.110.086165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanzonico P, Carrasquillo JA, Pandit-Taskar N, O'Donoghue JA, Humm JL, Smith-Jones P, et al. PET-based compartmental modeling of (124)I-A33 antibody: quantitative characterization of patient-specific tumor targeting in colorectal cancer. Eur J Nucl Med Mol Imag. 2015;42:1700–6. doi: 10.1007/s00259-015-3061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erdi YE, Macapinlac H, Larson SM, Erdi AK, Yeung H, Furhang EE, et al. Radiation dose assessment for I-131 therapy of thyroid cancer using I-124 PET imaging. Clin Pos Imag. 1999;2:41–6. doi: 10.1016/s1095-0397(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 25.Kolbert KS, Pentlow KS, Pearson JR, Sheikh A, Finn RD, Humm JL, et al. Prediction of absorbed dose to normal organs in thyroid cancer patients treated with 131I by use of 124I PET and 3-dimensional internal dosimetry software. J Nucl Med. 2007;48:143–9. [PubMed] [Google Scholar]
- 26.Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. New Engl J Med. 2013;368:623–32. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barendswaard EC, Humm JL, O'Donoghue JA, Sgouros G, Finn RD, Scott AM, et al. Relative therapeutic efficacy of (125)I- and (131)I-labeled monoclonal antibody A33 in a human colon cancer xenograft. J Nucl Med. 2001;42:1251–6. [PubMed] [Google Scholar]
- 28.Ruan S, O'Donoghue JA, Larson SM, Finn RD, Jungbluth A, Welt S, et al. Optimizing the sequence of combination therapy with radiolabeled antibodies and fractionated external beam. J Nucl Med. 2000;41:1905–12. [PubMed] [Google Scholar]
- 29.Barendswaard EC, O'Donoghue JA, Larson SM, Tschmelitsch J, Welt S, Finn RD, et al. 131I radioimmunotherapy and fractionated external beam radiotherapy: comparative effectiveness in a human tumor xenograft. J Nucl Med. 1999;40:1764–8. [PubMed] [Google Scholar]
- 30.Antoniw P, Farnsworth AP, Turner A, Haines AM, Mountain A, Mackintosh J, et al. Radioimmunotherapy of colorectal carcinoma xenografts in nude mice with yttrium-90 A33 IgG and Tri-Fab (TFM) Br J Cancer. 1996;74:513–24. doi: 10.1038/bjc.1996.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Emir E, Qureshi U, Dearling JL, Boxer GM, Clatworthy I, Folarin AA, et al. Predicting response to radioimmunotherapy from the tumor microenvironment of colorectal carcinomas. Cancer Res. 2007;67:11896–905. doi: 10.1158/0008-5472.CAN-07-2967. [DOI] [PubMed] [Google Scholar]
- 32.Orcutt KD, Rhoden JJ, Ruiz-Yi B, Frangioni JV, Wittrup KD. Effect of small-molecule-binding affinity on tumor uptake in vivo: a systematic study using a pretargeted bispecific antibody. Mol Cancer Ther. 2012;11:1365–72. doi: 10.1158/1535-7163.MCT-11-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDevitt MR, Chattopadhyay D, Jaggi JS, Finn RD, Zanzonico PB, Villa C, et al. PET imaging of soluble yttrium-86-labeled carbon nanotubes in mice. PLOS ONE. 2007;2:e907. doi: 10.1371/journal.pone.0000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orcutt KD, Nasr KA, Whitehead DG, Frangioni JV, Wittrup KD. Biodistribution and clearance of small molecule hapten chelates for pretargeted radioimmunotherapy. Mol Imaging Biol. 2011;13:215–21. doi: 10.1007/s11307-010-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol. 2010;76:S10–9. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beauregard JM, Hofman MS, Pereira JM, Eu P, Hicks RJ. Quantitative (177)Lu SPECT (QSPECT) imaging using a commercially available SPECT/CT system. Cancer Imaging. 2011;11:56–66. doi: 10.1102/1470-7330.2011.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pentlow KS, Finn RD, Larson SM, Erdi YE, Beattie BJ, Humm JL. Quantitative imaging of Yttrium-86 with PET: the occurrence and correction of anomalous apparent activity in high density regions. Clin Pos Imaging. 2000;3:85–90. doi: 10.1016/s1095-0397(00)00046-7. [DOI] [PubMed] [Google Scholar]
- 38.Beattie BJ, Finn RD, Rowland DJ, Pentlow KS. Quantitative imaging of bromine-76 and yttrium-86 with PET: a method for the removal of spurious activity introduced by cascade gamma rays. Med Phys. 2003;30:2410–23. doi: 10.1118/1.1595599. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther. 2009;8:2861–71. doi: 10.1158/1535-7163.MCT-09-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wittrup KD, Thurber GM, Schmidt MM, Rhoden JJ. Practical theoretic guidance for the design of tumor-targeting agents. Method Enzymol. 2012;503:255–68. doi: 10.1016/B978-0-12-396962-0.00010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yazaki PJ, Lee B, Channappa D, Cheung CW, Crow D, Chea J, et al. A series of anti-CEA/anti-DOTA bispecific antibody formats evaluated for pre-targeting: comparison of tumor uptake and blood clearance. Protein Eng Des Sel. 2013;26:187–93. doi: 10.1093/protein/gzs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu G, Hnatowich DJ. A semiempirical model of tumor pretargeting. Bioconjugate Chem. 2008;19:2095–104. doi: 10.1021/bc8002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.