Abstract
Background
There is a general perception in practice that a vascular supply should be used when large pieces of bone graft are used, particularly those greater than 6 cm in length for long-bone and large-joint reconstructions. However, the scientific source of this recommendation is not clear.
Questions/purposes
We wished to perform a systematic review to (1) investigate the origin of evidence for this 6-cm rule, and (2) to identify whether there is strong evidence to support the importance of vascularization for longer grafts and/or the lack of vascularization for shorter grafts.
Methods
Two systematic reviews were performed using SCOPUS and Medline, one for each research question. For the first research purpose, a review of studies from 1975 to 1983 matching article title (“bone” and “graft”) revealed 725 articles, none of which compared graft length. To address the second purpose, a review of articles before 2014 that matched “bone graft” AND (“vascularised” OR “vascularized”) AND (“non-vascularised” OR “non-vascularized”) revealed 633 articles, four met prespecified inclusion criteria and were evaluated qualitatively. MINORS ratings ranged from 16 to 18 of 24, and National Health and Medical Research Council [NHMRC] Evidence Hierarchy ratings ranged from III-2 (comparative studies without concurrent controls) to III-3 (comparative studies with concurrent controls).
Results
No evidence was found that clarified grafts longer than 6 cm should be vascularized. The first reference to the 6-cm rule cites articles that do not provide strong evidence for the rule. Of the four articles found in the second systematic review, none examined osseous union of vascularized and nonvascularized grafts with respect to length. One study (III-3, MINORS 18 of 24) of fibular grafts to various limb defects found that vascularization made no difference to union rate or time to union. Vascularized grafts were more likely to require surgical revision for wound breakdown, nonunion, graft fracture, or mechanical problems (hazard ratio [HR], 5.97, p = 0.008) and grafts smaller than 10 cm had fewer complications requiring revision (HR, 0.88; p = 0.03). Three studies (III-2 to III-3, MINORS 16 to 18 of 24) that examined fibular grafts to the femoral head found that vascularized grafts had superior Harris hip and pain scores. Two of the three articles showed that vascularization was associated with superior radiologic measures of collapse progression.
Conclusions
No compelling evidence was found to illuminate the origin of the 6-cm rule for vascularized bone grafts, or that such a rule is based on published research. The evidence we found for grafts to long-bone defects suggested that vascularization might increase the risk of complications that require a surgical revision without increasing union rates or time to union. For large joints, vascularization may result in better functional scores and pain scores, while the evidence that they improve radiologic measures of progression is mixed. There were no studies of long-bone or large-joint reconstructions that examined the role of length with respect to osseous union. We suggest that future studies should present data for graft lengths quantitatively and with individual data points rather than categories of length ranges.
Level of Evidence
Level III, therapeutic study.
Introduction
In the management of bony defects, autologous bone grafts can be used as the mechanical structure for reconstruction to restore aesthetics and function. The decision regarding whether to vascularize a graft for use in long-bone or large-joint reconstructions requires consideration of the benefits and risks. The theoretical advantages of the vascularized bone graft (VBG) over the nonvascularized bone graft (NVBG) are related to the provision of nutrients to the deep structures of the graft [5]. VBGs are used to minimize graft resorption and subsequent mechanical failure and to prevent infection. However, VBGs are more technically demanding, result in longer surgical procedures, cause greater donor site morbidity, and may be more difficult to match to the geometry of the recipient site [48]. It is undesirable to subject a patient to the additional stress of a VBG if the defect can be satisfactorily repaired with a NVBG. However, the use of a NVBG where a VBG is more appropriate could result in graft failure and additional surgery.
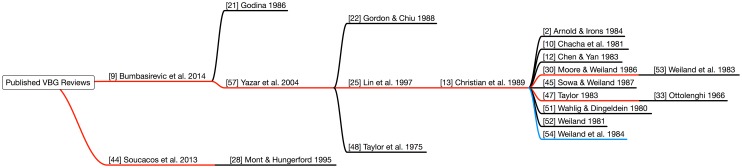
In autologous bone grafts, one factor in the decision to use a VBG is the length of the defect to be repaired. For longer grafts, the distance over which creeping substitution must take place also increases. This may translate to a longer or incomplete recovery. In practice and in modern research studies, defects larger than 5 to 7 cm generally have not been considered candidates for NVBGs [9, 20, 44]. However, the origin of the 6-cm rule is not readily apparent in those studies, which point to an array of articles that do not present relevant evidence (Fig. 1). A review of fibular VBGs published in 2014 recommends that defects greater than 6 cm be treated with VBGs [9]. However, the two references in the article used to support that argument [21, 57] reveal no origin for this recommendation. The first of the two [21] does not study or reference graft lengths, and the second [57] recommends VBGs for greater than 6-cm grafts based on an additional three articles [22, 25, 48]. A look at those three articles finds that one [25] also recommends VBGs for greater than 6-cm grafts and cites a case series of eight VBGs for tibial defects [13] and subsequently references numerous sources of low relevance [2, 10, 11, 30, 32, 45, 47, 51–54]. Another of the three articles [22] is a case series of 14 VBGs used for tibial defects, although only four grafts exceed 3 cm. The final article cited is the report by Taylor et al. [48] of the first human VBG in which it is suggested that VBGs might be indicated for large defects. This is based on outcomes of NVBGs published in a textbook [14] 12 years before, in which it is noted that “hemicylindrical tibial slide procedures… [and] free bone graft onlay or interposition, usually obtained from the iliac crest… have a higher failure rate if the defect is greater than 6 cm” [48]. In seeking evidence for the need for VBGs greater than a specific length, two obstacles frequently are encountered: the citation of secondary sources and qualitative descriptors of length. Similar to a 2014 review [9], a 2013 review of VBGs states that defects greater than 5 to 6 cm require VBGs [44]. However the source, a review article does not mention a centimeter-based rule for bone grafts [28].
Fig. 1.
Review articles supporting the 6-cm rule relied on evidence removed by up to 6o of separation. The blue line represents primary articles directly comparing vascular bone grafts (VBGs) and nonvascularized bone grafts (NVBGs). The red lines represent articles citing the 6-cm rule, and the black lines represent articles not directly comparing VBGs and NVBGs (Color figure online).
We therefore sought to perform a systematic review to (1) investigate the origin of evidence for this 6-cm rule and (2) to identify whether there is strong evidence to support the importance of vascularization for longer grafts and/or the lack of vascularization for shorter grafts.
Search Strategy and Criteria
Is There a Clear Origin of the 6-cm Rule for Vascularized Bone Grafts?
To identify all sources that may have contributed to the 6-cm rule, articles published between and including 1975, the year of the first published VBG [48] and 1983, the earliest reference to VBGs being superior at lengths greater than 6 cm [53], with the title constraint (“bone” AND “graft”), were searched for by one investigator (BA) on SCOPUS and Medline. Conference proceedings and bibliographies of articles that met inclusion criteria during this period also were hand-searched for references with titles that contained (“bone” AND “graft”). Letters and editorials were excluded, as were studies that did not directly compare VBGs and NVBGs. The search returned a combined 951 articles (Fig. 2) from SCOPUS (n = 633) and Medline (n = 318). Additional articles (n = 1) from hand searches were added and duplicates (n = 227) were removed. The articles (n = 725) were screened and excluded (n = 721) if there was no comparison between VBGs and NVBGs. Full-text was obtained for the remaining articles (n = 4) [5, 17, 29, 36] and those with no comparison of graft length were excluded (n = 4). There were no articles that met the inclusion criteria.
Fig. 2.
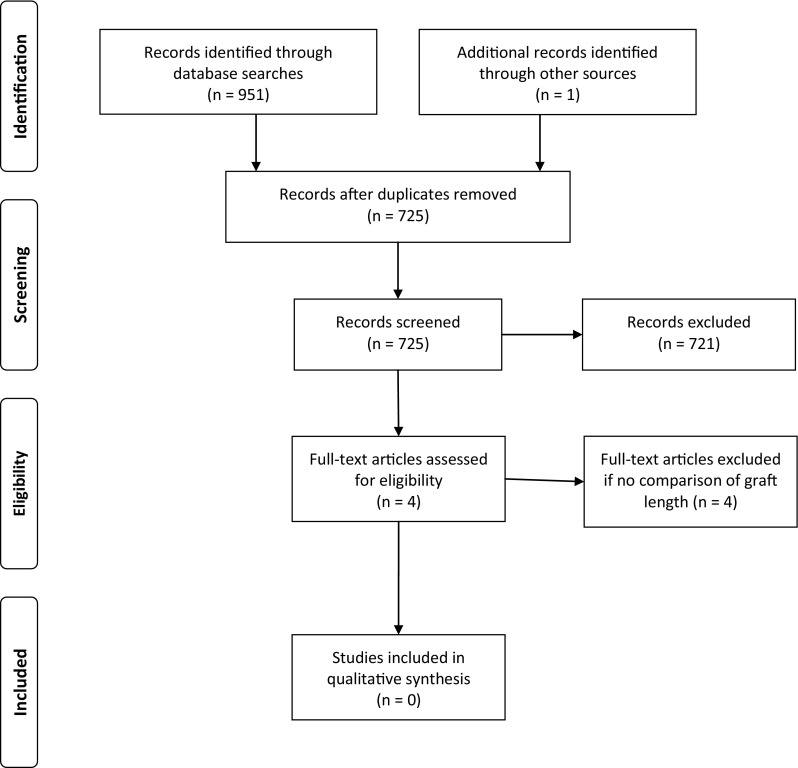
The PRISMA [27] diagram for the question “Is There a Clear Origin of the “6-cm” Rule for Vascularized Bone Grafts?” outlining the systematic review search process is shown (Color figure online).
What Evidence Supports the Need for Vascularization of Longer Bone Grafts?
A systematic search on SCOPUS and Medline for all times until 2014 was conducted using the following search strategy with all search fields included: “bone graft” AND (“vascularised” OR “vascularized”) AND (“non-vascularised” OR “non-vascularized”). One investigator (BA) performed the search and examined conference proceedings and bibliographies by hand to identify articles that also satisfied the Boolean search criteria above. Letters, editorials, review articles, animal studies, and histologic and mechanical analyses were excluded. Articles also were excluded if there was either no comparison, or reference of a comparison, between VBGs and NVBGs. The references of included articles also were examined for inclusion. This process was repeated until no additional articles were obtained. The details of the rate of union, bone length, strength, and graft type were extracted from the articles and tabulated in a spreadsheet for analysis.
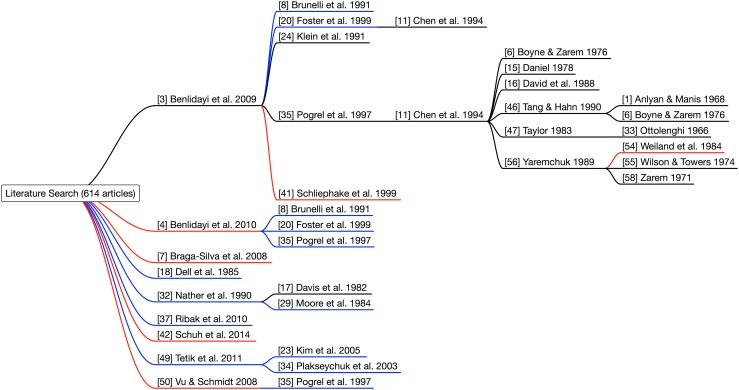
The search returned 614 articles (Fig. 3) from SCOPUS (n = 418) and Medline (n = 196) and additional articles (n = 21) identified in hand searches of articles and conference proceedings. Duplicates (n = 7) were removed and the remaining articles (n = 628) were screened and excluded (n = 598) if bone graft vascularity was not a focus of the paper. Full text was obtained for the remaining articles (n = 30). Animal studies, studies with histologic and mechanical analyses, mandibular studies, scaphoid studies, and those with no direct comparison of VBGs and NVBGs were excluded (n = 26) (Fig. 4) [1, 3, 4, 6–8, 11, 15–18, 20, 24, 29, 32, 33, 35, 37, 41, 46, 47, 50, 54–56, 58]. The remaining articles (n = 4) [23, 34, 42, 49] were analyzed qualitatively. The quality of the articles was evaluated using the MINORS scale [43] and National Health and Medical Research Council [NHMRC] Evidence Hierarchy [26]. MINORS ratings ranged from 16 to 18 of 24 (16, 16, 18, 18) and NHMRC Level of Evidence ranged from III-2 (n = 2) to III-3 (n = 2).
Fig. 3.
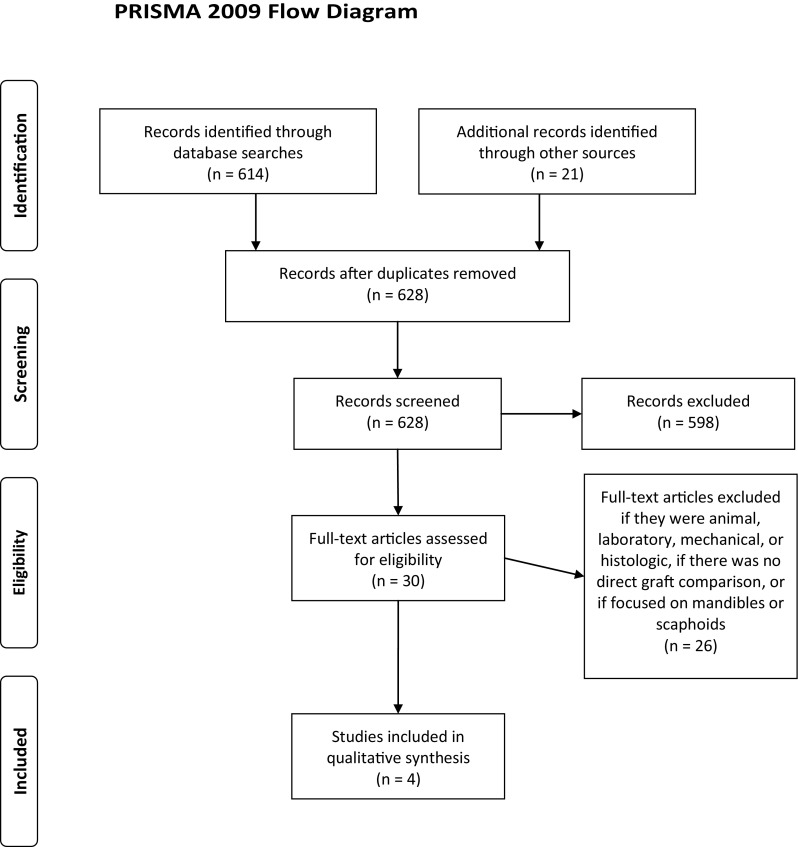
The PRISMA [27] diagram for the question “What Evidence Supports the Need for Vascularization of Longer Bone Grafts?” outlining the systematic review search process is shown (Color figure online).
Fig. 4.
The diagram shows our literature search for articles comparing vascularized bone grafts (VBGs) and nonvascularized bone grafts (NVBGs). The blue lines represent articles with evidence for the superiority of VBGs; the red lines represent articles showing nonsuperiority of VBGs; and the black lines represent articles where there was no direct comparison of VBGs and NVBGs (Color figure online).
Results
Is There a Clear Origin of the 6-cm Rule for Vascularized Bone Grafts?
We found no clear source in our systematic review supporting the idea that grafts longer than 6 cm need to be vascularized. The four articles that compared VBGs and NVBGs between 1975 and 1983 were all animal studies that made no comparison of graft length and were excluded [5, 17, 29, 36]. The earliest reference that VBGs “offer significant advantages over conventional treatment methods in selected patients with segmental bone defects greater than 6 cm” [53] from an article describing 41 VBGs for tumors and trauma in the extremities, therefore is not based on studies that compare VBGs and NVBGs
In a case series of two VBGs to tibial defects, Taylor et al. [48] suggested that VBGs are suited to longer bone defects, a claim that was not related to the outcome of the grafts. Weiland et al. [53], in their report of 41 VBGs to long bone defects published in 1983, stated that VBGs have advantages over NVBGs in defects larger than 6 cm. However, this statement is not based on the results of their study, there are no supporting references and no references cited consider graft. One month later Moore et al. [31] reported the results of 10 VBGs to various long-bone defects for tumors, cysts, and pseudarthroses, all 7.5 cm or longer, and concluded that VBGs are indicated only where graft length exceeds 6 cm. This recommendation was based on the 1980 report from Enneking et al. [19] of 40 NVBGs, all larger than 7.5 cm, to various long bones for tumors, in which 67% of patients achieved bony union. However, Enneking et al. [19] concluded that nonunion was not related to graft length.
What Evidence Supports the Need for Vascularization of Longer Bone Grafts?
The evidence is contradictory in terms of whether there is a clear benefit of vascularization for longer bone-graft segments (Table 1). Of the four articles identified, none presented evidence for a difference between VBGs and NVBGs based on length. The articles, including one comparison of fibular grafts to extremities and three comparisons of fibular grafts to the femoral head [23, 34, 42, 49] did not provide strong evidence that VBGs are superior to NVBGs as graft length increases, nor that they are inferior to NVBGs.
Table 1.
Summary of data for vascularization of longer bone grafts
| Study | Evidence Level | MINORS Score | Length-based differences presented? | Group favored | Was there a specific length where a difference arose (details)? | Donor (number, details) | Recipient (number, details) | Mean months of followup (range) | Findings indirectly related to length |
|---|---|---|---|---|---|---|---|---|---|
| Kim et al. [23] | III-2 | 18 | No | Neither | No (all grafts were to femoral head and no length details were presented) | VBG (23 fibula) NVBG (23 fibula) |
Femoral head (46) | 50 (36–67) | For all lengths (size of femoral head/neck), VBGs had less radiographic compression, collapse and dome depression |
| Plakseychuk et al. [34] | III-2 | 16 | No | Neither | No (all grafts were to femoral head and no length details were presented) | VBG (50 fibula) NVBG (50 fibula) |
Femoral head (100) | 60 (36–96) | For all lengths (size of femoral head/neck), VBGs had better outcomes on functional (Harris hip) questionnaire and collapse status on plain x-ray |
| Schuh et al. [42] | III-3 | 18 | No | Neither | No (outcomes related to length were not analyzed separately for VBGs and NVBGs) | Fibula (52) | VBG (1 humerus, 3 radius/ulna, 6 femur, 11 tibia, 5 foot) NVBG (2 humerus, 4 radius/ulna, 1 femur, 19 tibia, 0 foot) |
52 (12–259) | For all lengths: - VBG mean 14.7cm (8–27) - NVBG mean 15.2cm (5–28) VBGs were more likely to require revision (HR 5.97, p=0.008) All grafts <10cm were less likely (HR 0.88, p=0.03) to have a complication requiring revision (nonunion, wound breakdown, graft fracture, mechanical) |
| Tetik et al. [49] | III-3 | 16 | No | Neither | No (all grafts were to femoral head and no length details were presented) | VBG (11 fibula) NVBG (15 fibula) |
Femoral head (26) | 33 (12–114) | For all lengths (size of femoral head/neck), VBGs had better outcomes on functional (Harris hip) questionnaire and visual pain scale |
NVBG = nonvascularized bone graft; VBG = vascularized bone graft.
The articles provided inconclusive evidence for the existence of a relationship between length and the need for vascularization. One study (Evidence Hierarchy III-3, MINORS score 18 of 24), which examined 52 fibular grafts to various limb destinations for musculoskeletal tumors, found no difference between the union rates and time to union for VBGs and NVBGs [42]. That study did not examine length-based outcomes separately for VBGs and NVBGs. VBGs (mean, 14.7 cm) were more likely to require surgical revision than NVBGs (mean, 15.2 cm) (hazard ratio [HR], 5.97; p = 0.008) and for all grafts VBGs (mean, 14.7 cm; range, 8–27 cm), and NVBGs (mean, 15.2 cm; range, 5–28 cm), those less than 10 cm were less likely (HR, 0.88; p = 0.03) to have a complication requiring revision (nonunion, wound breakdown, graft fracture, mechanical).
The other three studies examined grafts to the femoral head and showed that VBGs had better radiographic [23, 34, 49] and functional outcomes [34, 49]. Kim et al. [23] studied 46 fibular grafts to the femoral head (Evidence Hierarchy III-3, MINORS score 18 of 24) and did not differentiate grafts based on length. VBGs were found to have less dome depression (2.8 mm versus 4.3 mm; p < 0.05), radiographic progression rates (13/23 versus 20/23; p < 0.05), and radiographic collapse rates (8/23 versus 16/23; p < 0.05) than NVBGs. Functional outcomes (Harris hip score) improved for 70% of VBGs and 35% of NVBGs (p < 0.05). Plakseychuk et al. [34] studied 100 fibular grafts to the femoral head (Evidence Hierarchy III-2, MINORS score 16 of 24) and did not examine grafts based on length. VBGs had a higher rate of improved functional outcome on Harris hip score (70% versus 36%; p < 0.05) and lower collapse rate on plain radiographs (14% versus 70%). Tetik et al. [49] studied 26 fibular grafts to the femoral head (Evidence Hierarchy III-3, MINORS score 16 of 24) and did not examine grafts based on length. There were no radiologic differences between groups, but VBGs had higher Harris hip scores (83.1 versus 61; 2 p < 0.05) and lower VAS scores for pain (2.8 versus 4.2; p < 0.05).
Discussion
With decision-making in surgery, it is important to understand the origin of standards of practice and to be aware of the evidence. In the decision to use a vascular supply for a bone graft, it is beneficial to know how length arguments first entered the discussion and what studies have contributed to the discourse. From the studies we examined, we found a mix of evidence that is difficult to readily synthesize. Part of the difficulty arises from graft length being a secondary focus of the articles, where length subgroups are created without presentation of original length data. We found that the idea that grafts longer than 6 cm need vascularization was based on insubstantial evidence. Additionally, we found that it is not clear that there is a need for vascularization of longer bone grafts. The concept that the performance of VBGs and NVBGs diverges as length increases remains plausible, however such a difference cannot be interpreted from the current literature.
Our study has several limitations. First, the number of clinical studies that examine length is small and no studies examined how VBGs and NVBGs differed with respect to length, which was the primary research question. Second, all studies were Level III-2 or III-3 and the quality of studies did not exceed MINORS scores of 18 of 24. Third, one investigator did the reviews. Fourth, the search criteria might not fully capture all articles that compare VBGs and NVBGs.
We found no evidence to support the idea that grafts longer than 6 cm require vascularization. Between the first VBG case series of two fibular grafts for trauma [48] and the first claim that 6-cm VBGs outperform NVBGs that appears in a series of 41 VBGs to long bone defects [53], there are only two studies that discuss graft success in the context of length. The first is a study of 53 particulate mesh grafts to the mandible that mentions in the introduction that long mandibular grafts tend to fail [6], and the second is a case series of 40 NVBGs for tumors in multiple anatomic locations that concludes graft success is unrelated to length [19]. It is our understanding that a statement by Weiland et al. [53] in the 1983 article on 41 VBGs to long bone defects in which VBGs were said to “offer significant advantages… [for] defects greater than 6 cm” has been interpreted to mean that VBGs have been proven to be superior to NVBGs at those lengths. The 6-cm rule has proliferated from that point and modern reviews of grafts for long-bone and large-joint reconstructions decades later still quote the rule [9, 44]. This rule may have biased subsequent studies that examine length, by encouraging the use of length categories that use 6 cm as a boundary, rather than analyzing the data without prejudice. Studies that seek to find a length at which VBG and NVBG performance differs will need to provide individual data points to support that there is a significant difference at that length.
There was no compelling evidence that VBGs produce better outcomes for longer bone grafts. Four studies of fibular and iliac crest grafts to the mandible [20, 35, 41, 50] and two studies of grafts from the iliac crest and radius to the scaphoid [7, 37] that directly compared VBGs and NVBGs were identified and excluded from the search. These studies are inconclusive with respect to whether VBGs outperform NVBGs at increasing length, and are no basis to make clinical recommendations regarding long-bone and large-joint defects. However, two of the mandibular studies [20, 35] are unique in that they compare VBGs and NVBGs at different length categories; a feature needed in future long-bone and large-joint studies. In these studies, the grafts are divided into categories (< 6 cm, 6–10 cm, 10–14 cm, > 14 cm [20] and < 6 cm, 6–9 cm, 9–12 cm, > 12 cm [35]) and VBGs and NVBGs are compared. Interestingly, one study identified a difference only for grafts greater than 6 cm, but not at individual graft categories such as 10 to 14 cm [20]. The other study found that as length increased for NVBGs, the rate of failure increased, but did not present the failure rate as VBG length increased [35]. These two examples suggest that creating length categories may (1) result in misleading interpretations of the data, or (2) result in incomplete presentation of data. These studies highlight the importance of presentation of individual datum in future studies of long-bone and large-joint reconstructions.
The data we examined were insufficient to show a relationship between the length of a graft and the need for a vascular supply. It is plausible that there is a difference and future efforts should be directed at answering this important question. We suggest that comparative prospective studies with groups that have equivalent baseline characteristics that present outcomes with respect to individual lengths would be beneficial.
The 6-cm rule has been cited for decades; however, the evidence for using VBGs rather than NVBGs for longer defects is limited. Existing studies have used various outcome measures to evaluate graft success and common trends include extrapolating relatively short-term data to long-term results and broad claims based on small subsets of grafts. It is unclear, given the different mechanisms by which vascularized and nonvascularized grafts heal, whether one approach or the other is better. Operative time, donor site morbidity, and the burden of microvascular revision surgery are problems associated with vascularized grafts. It is not well established that the long-term success of vascularized grafts is superior to that expected with nonvascularized grafts. Similarly, the benefit of the vascularization of increasingly small grafts remains unclear. Technical success in vascularization of other small defects has been described, particularly in the setting of small osteochondral flaps, suggesting that outcome studies may be forthcoming [38–40]. Consideration of the benefits and risks remains important when grafting to defects of all lengths. Without a robust analysis of the viability of NVBGs at different lengths, there is a risk that the 6-cm rule, which is unsupported by evidence, will result in the use of vascularized grafts where they may not be necessary, perhaps exposing patients to larger and riskier surgical procedures. Comparative clinical trials between larger and shorter vascularized and nonvascularized bone grafts need to be performed to answer this important question more definitively.
Acknowledgments
We thank Monash University for assistance in accessing the articles used in this paper.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
This work was performed at Frankston Hospital, Frankston, Victoria, Australia.
References
- 1.Anlyan AJ, Manis JR. Re-evaluation of bone chip grafts for mandibular defects. Am J Surg. 1968;116:606–609. doi: 10.1016/0002-9610(68)90401-7. [DOI] [Google Scholar]
- 2.Arnold PG, Irons GB. Lower-extremity muscle flaps. Orthop Clin North Am. 1984;15:441–449. [PubMed] [Google Scholar]
- 3.Benlidayi ME, Gaggl A, Buerger H, Kürkcü M, Ünlügenç H, Önal D, Polat S, Sencar L. Comparison of vascularized osteoperiosteal femur flaps and nonvascularized femur grafts for reconstruction of mandibular defects: an experimental study. J Oral Maxillofac Surg. 2009;67:1174–1183. doi: 10.1016/j.joms.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Benlidayi ME, Gaggl A, Bürger H, Brandner C, Kürkcü M, Ünlügenç H. Comparative study of the osseointegration of dental implants after different bone augmentation techniques: vascularized femur flap, non-vascularized femur graft and mandibular bone graft. Clin Oral Implants Res. 2010;22:594–599. doi: 10.1111/j.1600-0501.2010.02013.x. [DOI] [PubMed] [Google Scholar]
- 5.Berggren A, Weiland AJ, Ostrup LT. Bone scintigraphy in evaluating the viability of composite bone grafts revascularized by microvascular anastomoses, conventional autogenous bone grafts, and free non-revascularized periosteal grafts. J Bone Joint Surg Am. 1982;64:799–809. [PubMed] [Google Scholar]
- 6.Boyne PJ, Zarem H. Osseous reconstruction of the resected mandible. Am J Surg. 1976;132:49–53. doi: 10.1016/0002-9610(76)90289-0. [DOI] [PubMed] [Google Scholar]
- 7.Braga-Silva J, Peruchi FM, Moschen GM, Gehlen D, Padoin AV. A Comparison of the use of distal radius vascularised bone graft and non-vascularised iliac crest bone graft in the treatment of non-union of scaphoid fractures. J Hand Surg Eur. 2008;33:636–640. doi: 10.1177/1753193408090400. [DOI] [PubMed] [Google Scholar]
- 8.Brunelli G, Vigasio A, Battiston B, Di Rosa F, Brunelli G., Jr Free microvascular fibular versus conventional bone grafts. Int Surg. 1991;76:33–42. [PubMed] [Google Scholar]
- 9.Bumbasirevic M, Stevanovic M, Bumbasirevic V, Lesic A, Atkinson HD. Free vascularised fibular grafts in orthopaedics. Int Orthop. 2014;38:1277–1282. doi: 10.1007/s00264-014-2281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chacha PB, Ahmed M, Daruwalla JS. Vascular pedicle graft of the ipsilateral fibula for non-union of the tibia with a large defect: an experimental and clinical study. J Bone Joint Surg Br. 1981;63:244–253. doi: 10.1302/0301-620X.63B2.7217150. [DOI] [PubMed] [Google Scholar]
- 11.Chen YB, Chen HC, Hahn LH. Major mandibular reconstruction with vascularized bone grafts: indications and selection of donor tissue. Microsurgery. 1994;15:227–237. doi: 10.1002/micr.1920150403. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZW, Yan W. The study and clinical application of the osteocutaneous flap of fibula. Microsurgery. 1983;4:11–16. doi: 10.1002/micr.1920040107. [DOI] [PubMed] [Google Scholar]
- 13.Christian EP, Bosse MJ, Robb G. Reconstruction of large diaphyseal defects, without free fibular transfer, in Grade-IIIB tibial fractures. J Bone Joint Surg Am. 1989;71:994–1004. [PubMed] [Google Scholar]
- 14.Crenshaw AH, editor. Campbell’s Operative Orthopaedics. St Louis, MO: C.V. Mosby Company; 1963. [Google Scholar]
- 15.Daniel RK. Mandibular reconstruction with free tissue transfers. Ann Plast Surg. 1978;1:346–371. doi: 10.1097/00000637-197807000-00002. [DOI] [PubMed] [Google Scholar]
- 16.David DJ, Tan E, Katsaros J, Sheen R. Mandibular reconstruction with vascularized iliac crest: a 10-year experience. Plast Reconstr Surg. 1988;82:792–803. doi: 10.1097/00006534-198811000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Davis PK, Mazur JM, Coleman GN. A torsion strength comparison of vascularized and nonvascularized bone grafts. J Biomech. 1982;15:875–880. doi: 10.1016/0021-9290(82)90053-7. [DOI] [PubMed] [Google Scholar]
- 18.Dell PC, Burchardt H, Glowczewskie FP., Jr A roentgenographic, biomechanical, and histological evaluation of vascularized and non-vascularized segmental fibular canine autografts. J Bone Joint Surg Am. 1985;67:105–112. [PubMed] [Google Scholar]
- 19.Enneking WF, Eady JL, Burchardt H. Autogenous cortical bone grafts in the reconstruction of segmental skeletal defects. J Bone Joint Surg Am. 1980;62:1039–1058. [PubMed] [Google Scholar]
- 20.Foster RD, Anthony JP, Sharma A, Pogrel MA. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck. 1999;21:66–71. doi: 10.1002/(SICI)1097-0347(199901)21:1<66::AID-HED9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78:285–292. doi: 10.1097/00006534-198609000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Gordon L, Chiu EJ. Treatment of infected non-unions and segmental defects of the tibia with staged microvascular muscle transplantation and bone-grafting. J Bone Joint Surg Am. 1988;70:377–386. [PubMed] [Google Scholar]
- 23.Kim SY, Kim YG, Kim PT, Ihn JC, Cho BC, Koo KH. Vascularized compared with nonvascularized fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Joint Surg Am. 2005;87:2012–2018. doi: 10.2106/JBJS.D.02593. [DOI] [PubMed] [Google Scholar]
- 24.Klein L, Stevenson S, Shaffer JW, Davy D, Goldberg VM. Bone mass and comparative rates of bone resorption and formation of fibular autografts: comparison of vascular and nonvascular grafts in dogs. Bone. 1991;12:323–329. doi: 10.1016/8756-3282(91)90018-E. [DOI] [PubMed] [Google Scholar]
- 25.Lin CH, Wei FC, Levin LS, Su JI, Fan KF, Yeh WL, Hsu DT. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99:1656–1665. doi: 10.1097/00006534-199705010-00028. [DOI] [PubMed] [Google Scholar]
- 26.Merlin T, Weston A, Tooher R. Extending an evidence hierarchy to include topics other than treatment: revising the Australian “levels of evidence”. BMC Med Res Methodol. 2009;9:34. doi: 10.1186/1471-2288-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J. Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Moore JB, Mazur JM, Zehr D, Davis PK, Zook EG. A biomechanical comparison of vascularized and conventional autogenous bone grafts. Plast Reconstr Surg. 1984;73:382–386. doi: 10.1097/00006534-198403000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Moore JR, Weiland AJ. Free vascularized bone and muscle flaps for osteomyelitis. Orthopedics. 1986;9:819–824. doi: 10.3928/0147-7447-19860601-08. [DOI] [PubMed] [Google Scholar]
- 31.Moore JR, Weiland AJ, Daniel RK. Use of free vascularized bone grafts in the treatment of bone tumors. Clin Orthop Relat Res. 1983;175:37–44. [PubMed] [Google Scholar]
- 32.Nather A, Goh JC, Lee JJ. Biomechanical strength of non-vascularised and vascularised diaphyseal bone transplants: an experimental study. J Bone Joint Surg Br. 1990;72:1031–1035. doi: 10.1302/0301-620X.72B6.2246285. [DOI] [PubMed] [Google Scholar]
- 33.Ottolenghi CE. Massive osteoarticular bone grafts: transplant of the whole femur. J Bone Joint Surg Br. 1966;48:646–659. [PubMed] [Google Scholar]
- 34.Plakseychuk AY, Kim SY, Park BC, Varitimidis SE, Rubash HE, Sotereanos DG. Vascularized compared with nonvascularized fibular grafting for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85:589–596. doi: 10.2106/00004623-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Pogrel MA, Podlesh S, Anthony JP, Alexander J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg. 1997;55:1200–1206. doi: 10.1016/S0278-2391(97)90165-8. [DOI] [PubMed] [Google Scholar]
- 36.Puckett CL, Hurvitz JS, Metzler MH, Silver D. Bone formation by revascularized periosteal and bone grafts, compared with traditional bone grafts. Plast Reconstr Surg. 1979;64:361–365. doi: 10.1097/00006534-197909000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Ribak S, Medina CE, Mattar R, Jr, Ulson HJ, de Resende MR, Etchebehere M. Treatment of scaphoid nonunion with vascularised and nonvascularised dorsal bone grafting from the distal radius. Int Orthop. 2010;34:683–688. doi: 10.1007/s00264-009-0862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rozen WM, Ek EW. Re: The osteochondral dilemma: review of current management and future trends. ANZ J Surg. 2014;84:695–696. doi: 10.1111/ans.12722. [DOI] [PubMed] [Google Scholar]
- 39.Rozen WM, Niumsawatt V, Leong JC, Ek EW. The vascular basis of the hemi-hamate osteochondral free flap: Part 2. Surgical anatomy and clinical application. Surg Radiol Anat. 2013;35:595–608. doi: 10.1007/s00276-012-1072-2. [DOI] [PubMed] [Google Scholar]
- 40.Rozen WM, Niumsawatt V, Ross R, Leong JC, Ek EW. The vascular basis of the hemi-hamate osteochondral free flap: Part 1. Vascular anatomy and clinical correlation. Surg Radiol Anat. 2013;35:585–594. doi: 10.1007/s00276-013-1098-0. [DOI] [PubMed] [Google Scholar]
- 41.Schliephake H, Schmelzeisen R, Husstedt H, Schmidt-Wondera LU. Comparison of the late results of mandibular reconstruction using nonvascularized or vascularized grafts and dental implants. J Oral Maxillofac Surg. 1999;57:944–950; discussion 950-951. [DOI] [PubMed]
- 42.Schuh R, Panotopoulos J, Puchner SE, Willegger M, Hobusch GM, Windhager R, Funovics PT. Vascularised or non-vascularised autologous fibular grafting for the reconstruction of a diaphyseal bone defect after resection of a musculoskeletal tumour. Bone Joint J. 2014;96:1258–1263. doi: 10.1302/0301-620X.96B9.33230. [DOI] [PubMed] [Google Scholar]
- 43.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 44.Soucacos PN, Kokkalis ZT, Piagkou M, Johnson EO. Vascularized bone grafts for the management of skeletal defects in orthopaedic trauma and reconstructive surgery. Injury. 2013;44(suppl 1):S70–75. doi: 10.1016/S0020-1383(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 45.Sowa DT, Weiland AJ. Clinical applications of vascularized bone autografts. Orthop Clin North Am. 1987;18:257–273. [PubMed] [Google Scholar]
- 46.Tang YB, Hahn LJ. Major mandibular reconstruction with vascularized bone graft. J Formos Med Assoc. 1990;89:34–40. [PubMed] [Google Scholar]
- 47.Taylor GI. The current status of free vascularized bone grafts. Clin Plast Surg. 1983;10:185–209. [PubMed] [Google Scholar]
- 48.Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft: a clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55:533–544. doi: 10.1097/00006534-197505000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Tetik C, Başar H, Bezer M, Erol B, Ağir I, Esemenli T. Comparison of early results of vascularized and non-vascularized fibular grafting in the treatment of osteonecrosis of the femoral head. Acta Orthop Traumatol Turc. 2011;45:326–334. doi: 10.3944/AOTT.2011.2259. [DOI] [PubMed] [Google Scholar]
- 50.Vu DD, Schmidt BL. Quality of life evaluation for patients receiving vascularized versus nonvascularized bone graft reconstruction of segmental mandibular defects. J Oral Maxillofac Surg. 2008;66:1856–1863. doi: 10.1016/j.joms.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 51.Wahlig H, Dingeldein E. Antibiotics and bone cements: experimental and clinical long-term observations. Acta Orthop Scand. 1980;51:49–56. doi: 10.3109/17453678008990768. [DOI] [PubMed] [Google Scholar]
- 52.Weiland AJ. Current concepts review: vascularized free bone transplants. J Bone Joint Surg Am. 1981;63:166–169. [PubMed] [Google Scholar]
- 53.Weiland AJ, Moore JR, Daniel RK. Vascularized bone autografts: experience with 41 cases. Clin Orthop Relat Res. 1983;174:87–95. [PubMed] [Google Scholar]
- 54.Weiland AJ, Phillips TW, Randolph MA. Bone grafts: a radiologic, histologic, and biomechanical model comparing autografts, allografts, and free vascularized bone grafts. Plast Reconstr Surg. 1984;74:368–379. doi: 10.1097/00006534-198409000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Wilson JS, Towers JF. Mandibular reconstruction. Proc R Soc Med. 1974;67:603–607. [PMC free article] [PubMed] [Google Scholar]
- 56.Yaremchuk MJ. Vascularized bone grafts for maxillofacial reconstruction. Clin Plast Surg. 1989;16:29–39. [PubMed] [Google Scholar]
- 57.Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114:1457–1466. doi: 10.1097/01.PRS.0000138811.88807.65. [DOI] [PubMed] [Google Scholar]
- 58.Zarem HA. Current concepts in reconstructive surgery in patients with cancer of the head and neck. Surg Clin North Am. 1971;51:149–173. doi: 10.1016/s0039-6109(16)39338-0. [DOI] [PubMed] [Google Scholar]