FIGURE 4.
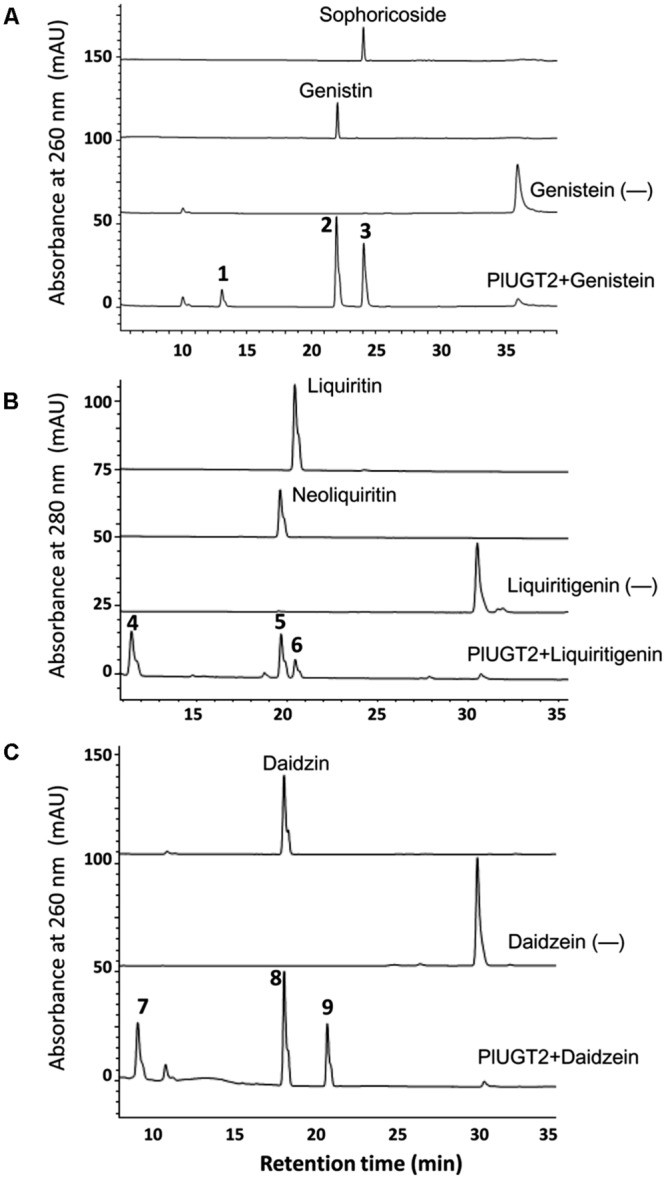
High-performance liquid chromatography (HPLC) analysis of the products from the in vitro reactions of the recombinant PlUGT2 with genitein (A), liquiritigenin (B), and daidzein (C). PlUGT2 was able to converted these (iso)flavone aglycones to yield their respective 4′,7-O-diglucosides (peaks 1, 4, 7), 7-O-mono-glucosides (peaks 2, 5, 8), and 4′-O-mono-glucosides (peaks 3, 6, 9). (-) Indicates control reactions without the addition of PlUGT2. Peak 1, genitein 4′,7-O-diglucoside; peak 2, genitin (genitein 7-O-glucoside); peak 3, sophoricoside (genitein 4′-O-glucoside); peak 4, liquiritigenin 4′,7-O-diglucoside; peak 5, neoliquiritin (liquiritigenin 7-O-glucoside); peak 6, liquiritin (liquiritigenin 4′-O-glucoside); peak 7, daidzein 4′,7-O-diglucoside; peak 8, daidzin (daidzein 7-O-glucoside); peak 9, daidzein 4′-O-glucoside. The mass spectra of all the reaction products (peaks 1–9) were shown in Supplementary Figure S3, and their chemical structures are listed in the Figure 1.
