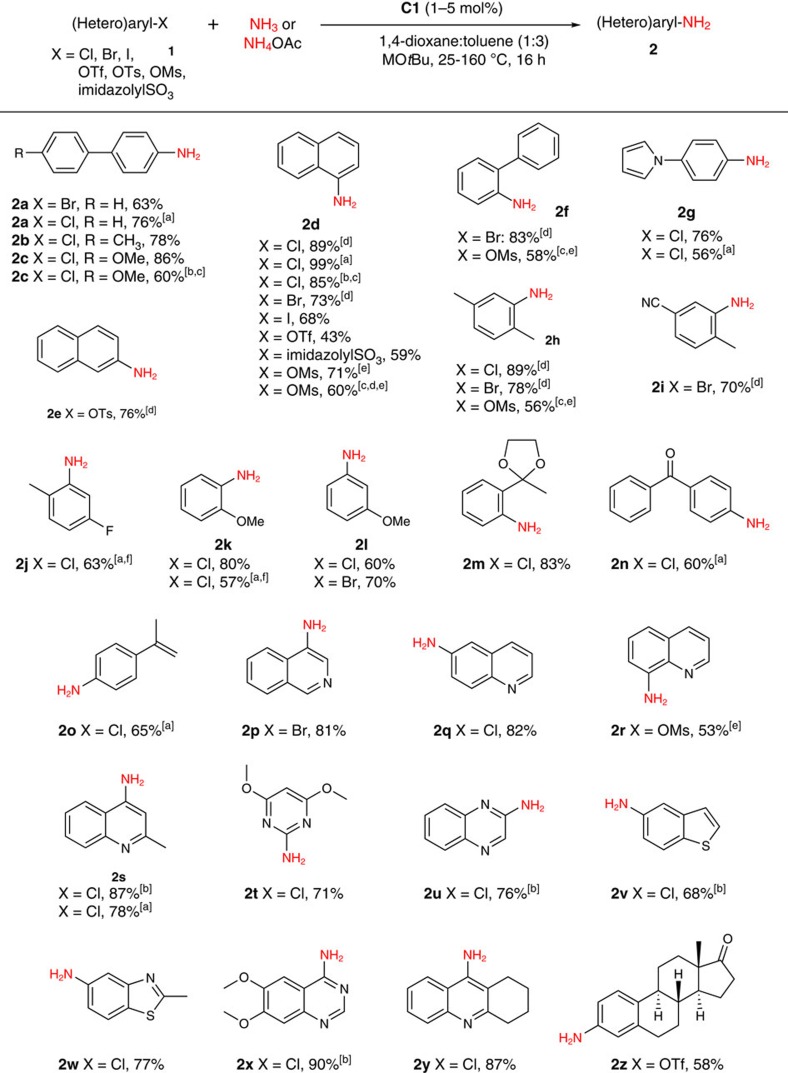
Figure 6. Scope of ammonia monoarylation using C1.
Unless stated otherwise, reactions were conducted employing C1 (1–5 mol%), MOtBu (M=Li or Na; 1.5–2.0 equiv), NH3 (from 0.5 M solutions in 1,4-dioxane; 3–8.3 equiv), in toluene for 16 h (unoptimized), with yields of isolated products reported; throughout, see the Supplementary Information for complete experimental details. [a] 110–160 °C for 5–30 min under microwave conditions using NH4OAc (5 equiv) and NaOtBu (6.5 equiv) in CPME. [b] Conducted using gaseous ammonia (114 psi initial pressure). [c] Yield on the basis of 1H NMR data relative to ferrocene as an internal standard. [d] 25 °C. [e] K3PO4 (6 equiv) used as base at 110 °C without toluene co-solvent. [f] Isolated as the N-tosylated derivative.