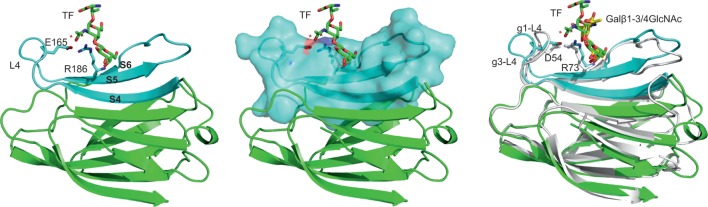
Figure 1.
Structural feature of galectin-3–TF interaction. Left panel: overall structure of galectin-3 CRD in complex with TF disaccharide (PDB 3AYA) (63). TF disaccharide (in stick model) binds to the CRD concave surf forms a E165-water-R186-water motif for TF recognition. Residues from strands S4–S6 (colored cyan) interact with the TF; L4 is the loop between S4 and S5. Middle panel: surface representation of strands S4–S6. Left panel: comparison between the structures of galectin-3 complex with the TF disaccharide and galectin-1 complex with Galβ1–3/4GlcNAc disaccharide (PDB 4XB1) (76). Galectin-3/TF is in green; galectin-1/Galβ1–3/4GlcNAc in white cartoon/yellow sticks. g1-L4 and g3-L4 are, respectively, the L4 loop for galectin-1 and galectin-3, which are found to adopt different conformations due to differences in the lengths and amino acid sequences of the two loops. D54 and R73 make up D54-water-R73-water motif that mediates ligand interactions.