Abstract
Childhood and adolescence coincide with rapid maturation and synaptic reorganization of distributed neural networks that underlie complex cognitive-affective behaviors. These regions, referred to collectively as the ‘social brain network’ (SBN) are commonly vulnerable to disruption from pediatric traumatic brain injury (TBI); however, the mechanisms that link morphological changes in the SBN to behavior problems in this population remain unclear. In 98 children and adolescents with mild to severe TBI, we acquired 3D T1-weighted MRIs at 2–8 weeks post-injury. For comparison, 33 typically developing controls of similar age, sex and education were scanned. All participants were assessed on measures of Theory of Mind (ToM) at 6 months post-injury and parents provided ratings of behavior problems at 24-months post-injury. Severe TBI was associated with volumetric reductions in the overall SBN package, as well as regional gray matter structural change in multiple component regions of the SBN. When compared with TD controls and children with milder injuries, the severe TBI group had significantly poorer ToM, which was associated with more frequent behavior problems and abnormal SBN morphology. Mediation analysis indicated that impaired theory of mind mediated the prospective relationship between abnormal SBN morphology and more frequent chronic behavior problems. Our findings suggest that sub-acute alterations in SBN morphology indirectly contribute to long-term behavior problems via their influence on ToM. Volumetric change in the SBN and its putative hub regions may represent useful imaging biomarkers for prediction of post-acute social cognitive impairment, which may in turn elevate risk for chronic behavior problems.
Keywords: childhood, brain injuries, social cognition, theory of mind, magnetic resonance imaging
Introduction
Deficits in social and behavioral functioning may be among the most profound and disabling consequences of pediatric traumatic brain injury (TBI). Research suggests that TBI may elevate risk for a range of social and affective problems, including depression, anxiety, interpersonal aggression, rule breaking and conduct problems (McKinlay et al., 2008, 2013; Li and Liu, 2013; Ryan et al., 2013). This association is supported by a disproportionately high prevalence of TBI in prisons and other custodial institutions (Kenny and Lennings, 2007; Williams et al., 2010; Davies et al., 2012; Moore et al., 2014), as well as population-based evidence that pediatric TBI is associated with a 4-fold increased risk for development of later mental disorder and coexisting offending behavior (Timonen et al., 2002). Despite evidence for a link between pediatric TBI and a range of maladaptive behaviors, the mechanisms underpinning this association are poorly understood.
The ability to engage in context appropriate behavior and achieve developmentally appropriate goals is dependent on socio-affective functions including Theory of Mind (ToM), a multi-dimensional construct that allows individuals to ascribe a variety of psychological states, such as intentions or emotions, to others and thereby understand and subsequently predict behaviors (Blakemore, 2008; Chertkoff Walz et al., 2010; Herbet et al., 2013). Although ToM shows rapid maturation through the preschool years (Wellman et al., 2001; Surian et al., 2007; Sodian, 2011), it also continues to show protracted development through late childhood and into mid adolescence, corresponding to the extended structural and functional maturation of the anatomically distributed social brain network (SBN), which includes the superior temporal sulcus (STS), fusiform gyrus (FG), temporal pole (TP), medial prefrontal cortex (mPFC), frontal pole (FP), orbitofrontal cortex (OFC), amygdala, insula, temporoparietal junction (TPG) and cingulate (Johnson et al., 2005; Choudhury et al., 2006; Adolphs, 2009; Dumontheil et al., 2010; Burnett et al., 2011). Although socio-affective skills subsumed by this network are vulnerable to disruption from pediatric TBI and are shown to elevate risk for maladaptive behavior (Dennis et al., 2012, 2013b; Ryan et al., 2013, 2014a, 2015b,c; Robinson et al., 2014), the prognostic significance of morphological change in this neural network is poorly understood.
Several studies involving advanced imaging modalities have documented persistent alterations to both gray and white matter (WM) networks following pediatric TBI (Wilde et al., 2005, 2006, 2012; Spanos et al., 2007; Fearing et al., 2008; Merkley et al., 2008; Bigler et al., 2010; Wu et al., 2010a,b; Beauchamp et al., 2011a). Levin et al. (2011) report that children with moderate-severe TBI demonstrate diffuse WM microstructural abnormalities, including alterations in areas implicated in social cognitive neural networks. Similarly, Dennis et al. (2013c) found that severe chronic pediatric TBI was associated with gray matter (GM) structural change in five large-scale functional brain networks, as well as regional brain volume loss in SBN regions that underlie higher order aspects of ToM. Despite the likely functional consequences of these diffuse structural brain changes, links between altered network morphology and maladaptive behaviors in this population are largely inconsistent across studies (Vasa et al., 2004; Max et al., 2012; Li and Liu, 2013).
One possibility is that structural changes in the SBN increase risk for behavior problems through impaired ToM. Consistent with this hypothesis, Dennis et al. (2013c) found that among children with chronic pediatric TBI, global volumetric reductions were associated with poorer performance on measures involving ToM—tasks of which are also known to predict social and behavioral problems in this population (Ryan et al., 2013, 2015c; Robinson et al., 2014). Collectively, these data suggest that volumetric reductions in the SBN may elevate risk for behavior problems through impaired ToM.
This study aimed to evaluate (i) the sub acute effect of pediatric TBI on SBN morphology; and (ii) prospective relationships between SBN volume, post-acute ToM and chronic behavioral problems. Specifically, we investigated whether ToM mediates the prospective relationship between structural change in the SBN and chronic behavioral difficulties. We had four hypotheses: (i) compared with healthy controls, and children with milder injuries, children with severe TBI would show volumetric reductions in the overall SBN package, as well as regional GM volumetric reductions in the individual component cortical regions of the SBN; (ii) children with severe TBI would show poorer ToM than controls and children with milder injuries; (iii) volumetric reductions in the SBN package would be related to poorer ToM and more frequent behavior problems; (iv) impaired ToM would mediate the prospective relationship between reduced SBN volume and chronic behavioral difficulties.
Methods
Participants
This study included 131 children: 98 survivors of TBI (68 males) and 33 typically developing (TD) children (24 males), group matched for age, sex and socio-economic status (SES). Children were recruited to participate in a larger longitudinal study, which aimed to investigate the psychosocial consequences of TBI (Anderson et al., 2013). All participants were ascertained between 2007 and 2010, and were aged between 5.3 and 15.4 years at time of recruitment. Children with TBI were recruited at time of injury, and represented consecutive admissions to The Royal Children’s Hospital (RCH), Melbourne, Australia. TD children were recruited from the community, through local schools chosen to provide a range of socio-economic backgrounds.
For the TBI group, inclusion criteria were: (i) age between 5.0 and 16 years at recruitment; (ii) documented evidence of closed head injury, including a period of altered consciousness or presence of at least two post-concussive symptoms; (iii) medical records sufficiently detailed to determine injury severity, including the Glasgow Coma Scale (GCS: Teasdale and Jennett, 1974), and neurological and radiological findings; (iv) no history of pre-injury neurological or developmental disorder (including learning or attentional disability, or autistic spectrum disorder), non-accidental injury, or previous TBI; and (v) no prior intervention for social impairment; (vi) English speaking. The TD group were required to meet inclusion criteria (i), (iv), (v) and (vi) above.
Participants with TBI were classified as; (i) mild (n = 53): GCS 13–15 on admission, no evidence of mass lesion on CT or clinical MRI, and no neurologic deficits; (ii) mild complex TBI (n = 13): GCS 13–15, evidence of mass lesion on CT or clinical MRI; (iii) moderate TBI (n = 22): GCS 9–12, and/or mass lesion or other evidence of specific injury on CT/MRI, and/or neurological impairment; (iv) severe TBI (n = 10): GCS 3–8, and/or mass lesion or other evidence of specific injury on CT/MRI, and/or neurological impairment.
The study was approved by The RCH Human Research Ethics Committee, and the Victorian Department of Education Ethics Committee. All parents gave their written, informed consent for children to participate in the study, and for extraction of clinical data from medical records at the time of recruitment.
Behavioral measures
Post-acute ToM: 6-months post-injury
Children were administered three measures to evaluate distinct aspects of ToM (Dennis et al., 2013c). The first measure, the Jack and Jill task (Dennis et al., 2012), assessed cognitive ToM, reflected in children’s understanding of false beliefs. Children were shown sequences of three cartoon frames on a computer screen. Each frame included a character (Jack and/or Jill), two hats (red and blue) and a ball. Frame A of the sequence showed Jack placing the ball in a hat, an event witnessed by Jill. In Frame B, Jack either dropped the ball further down into the hat, or switched the ball to the second hat. This event was sometimes witnessed, and sometimes not witnessed, by Jill. Frame C showed Jill ‘thinking’ about either the red or blue hat. Children responded ‘yes’ if Frame C represented what was now in Jill’s mind about the ball’s location, and they responded ‘no’ if it did not. The task measured cognitive ToM by presenting switched, unwitnessed trials that measured false belief, as compared with a series of switched, witnessed trials that measured true belief. Percent accuracy for switched, unwitnessed trials was the primary measure of cognitive ToM.
The Emotional and Emotive Faces Task (Dennis et al., 2013a) was used to assess affective ToM, or the child’s understanding of the difference between emotional expression (how a character actually feels) and emotive communication (the emotion a character expresses socially, which may be different from the felt emotion). Children were presented with short narratives that described a character in situations that were meant to evoke one of five basic emotions: happiness, sadness, disgust, fear and anger. In each vignette, a discrepancy existed between the emotion felt ‘inside’ and the character’s facial expression. In each trial, children were asked (i) how the character felt inside, and (ii) how the character looked on his/her face. Percent accuracy for emotive communication trials (i.e. ‘on his/her face’) was the primary measure of affective ToM.
The Ironic Criticism and Empathic Praise task (Dennis et al., 2013b) assesses conative ToM, or the child’s understanding of how indirect speech acts are used to influence the mental or emotional state of the listener. Children were presented with six pictured situations involving two children, one of whom was engaged in an activity and another who commented on their performance of the activity. The pictures were presented alongside a narrative, and an audiotape of the speaker’s utterances with neutral, ironic or empathic intonation. Children were told the goal of the child engaged in the activity (e.g. to build a tower), the outcome (e.g. ‘the tower was …’), the speaker’s character (e.g. ‘she liked to cheer people up’), and what the speaker said (e.g. ‘You made a great tower’). Children were asked two factual questions, two belief questions, and two intent questions. Percent accuracy for indirect speech acts, which reflected the understanding of belief and intent for empathic praise and ironic criticism conditions, was the primary measure of conative ToM.
Chronic behavior problems: 24-months post–injury
The Child Behavior Checklist (Achenbach and Rescorla, 2001) was completed by parents of participants based on child behavior over the previous 6-months. The respondent, in this case the primary caregiver, rates each of the 113 items as 0 = not true; 1 = somewhat or sometimes true; 2 = very true or often true. The Internalizing and Externalizing Problem scales are summed to generate the child behavior checklist (CBCL) Total Problems score, which was used in statistical analyses of outcome.
Structural MRI
Image acquisition
All children underwent a structural MRI research scan between 2 and 8 weeks post-injury (M = 39.25, SD = 27.64 days). MR images were acquired on a 3 T Siemens Trio scanner (Siemens Medical Systems, Erlangen, Germany) using a 32-Channel matrix head coil. High resolution T1-weighted structural MR images were acquired for each participant (TR = 1900 ms, FA = 9°, FOV = 256 × 224, 176 slices, slice thickness = 1.0 mm, in plane resolution 0.5 × 0.5 mm).
Brain morphometry measures
Morphometric analysis was conducted using the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu.au), which automatically segments and parcellates cortical GM and sub-cortical structures. This approach has previously been used in both adult (Warner et al., 2010) and pediatric TBI (Merkley et al., 2008). Full details regarding the analysis steps are documented elsewhere (Dale et al., 1999; Fischl et al., 2002, 2004; Yeates et al., 2004; Jovicich et al., 2006). FreeSurfer was used to determine volumes for total cortical volume (TCV), as well as cortical gray, white and corpus callosum (CC) volume. Using a probabilistic labeling algorithm that relies on gyral and sulcal information, the cortex was parcellated into anatomical regions of interest (ROIs) based on the Deskman-Killiany atlas and projected back into each subject’s native space. From this automated procedure, volume of the overall SBN package was computed by extracting GM volumes for the following regions bilaterally: STS, FG, frontal and TPs, mPFC, OFC, TPG, cingulate, insula and amygdala. The volume of each of these regions was summed to represent the overall SBN package. Estimated Total Intracranial Volume (ICV) was also extracted for use of ICV as a covariate.
Statistical analysis
ToM and behavior
Consistent with the approach of the test developers (Dennis et al., 2013c), individual scores on the ToM subtests were transformed to the same metric (i.e. % correct for ToM tasks), and a composite score was generated for each participant by averaging performance across the tasks. This yielded an overall indicator of ToM for use in subsequent mediation analyses. Calculation of the ToM composite score is in keeping with approach adopted in previous TBI research (Robinson et al., 2014), and is supported by the results of a recent factor analysis that suggested that these three measures loaded on a single factor (Dennis et al., 2013c; Robinson et al., 2014). Differences between groups in demographic and injury variables were examined using either analysis of variance (ANOVA) for continuous data or χ2 for categorical variables. Analysis of covariance (ANCOVA) using planned contrasts was used to investigate group differences for ToM and behavioral problems, covarying for SES [Australian and New Zealand Standard Classification of Occupations (ANZSCO) (McMillan et al., 2009)] and age.
MRI
For both global and regional analyses, volumetric data for the whole sample were analyzed using multivariate analysis of variance (MANOVA) with injury severity group membership as the between-subjects factor and ROI as the within-subjects factor.
Mediation analysis
We conducted mediation analysis across all TBI participants using bootstrapping, a non-parametric resampling procedure that constructs confidence intervals for the indirect effect of the proposed mediation (Hayes, 2009). We used the SPSS macro PROCESS to obtain estimates of the total, direct and indirect effects and associated 95% confidence intervals (CI95) using the recommended 5000 bootstrap samples (Hayes, 2013). If the intervals for a specific effect do not contain zero, the effect is considered statistically significant.
Although some research has recommended that mediator models be tested only in instances where independent (IV), dependent (DV), and mediating variables are mutually associated (Baron and Kenny, 1986), an emerging statistical literature has argued that an indirect, intervening influence on a DV may indeed exist, even when a direct association between the IV and DV does not (Hayes, 2009). In the context of this study where the mediation model rests on the theoretical assumption that SBN morphology predicts ToM, we computed mediation models for each regional measure of SBN morphology that was significantly associated with ToM. In each model, volume of each SBN region was entered as the independent variable, and the ToM composite was entered as a mediator of the effect of SBN volumes on behavioral problems (CBCL Total Score). ICV was entered as a covariate in each analysis. Age at testing was also added as a covariate in each model given the significant correlation between age at testing and the ToM composite.
Results
Demographic and injury variables
Table 1 presents demographic and injury characteristics for the TBI and TD control groups. Groups did not differ on sex, age at injury, or age at 6- or 24-month assessment. TD controls showed higher SES than the mild complicated TBI group, P = 0.04. SES was therefore used as a covariate in analyses of outcome. Because sex was unrelated to cognitive (P = 0.99), affective (P = 0.56) or conative ToM (P = 0.95), we did not adjust for sex in primary outcome analyses.
Table 1.
Demographic and injury characteristics of the sample
| Variable | TD Control | Mild TBI | Mild complicated TBI | Moderate TBI | Severe TBI |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| n | 33 | 53 | 13 | 22 | 10 |
| Male, n (%) | 20 (60.61) | 40 (75.47) | 8 (61.54) | 13 (59.10) | 7 (70.00) |
| Time between injury and MRI (days), M (SD) | 38.77 (21.84) | 37.62 (17.91) | 38.33 (19.34) | 57.31 (30.93) | |
| Age at 6-month assessment, M (SD) | 9.94 (2.95) | 11.25 (2.33) | 10.19 (2.46) | 11.08 (2.34) | 10.55 (3.30) |
| Age at 24-month assessment, M (SD) | 11.35 (2.88) | 12.62 (2.24) | 11.21 (2.58) | 12.82 (2.40) | 12.59 (3.05) |
| SES, M (SD)a,b | 75.86 (14.35) | 69.20 (20.06) | 56.69 (27.17) | 62.85 (24.65) | 74.84 (20.34) |
| Pre-TBI child adaptive behavior, M (SD) | 97.78 (17.64) | 96.92 (16.31) | 99.10 (10.35) | 97.44 (14.69) | |
| Injury characteristics | |||||
| Age at injury, M (SD) | 10.69 (2.35) | 9.65 (2.45) | 10.37 (2.47) | 10.33 (3.25) | |
| Lowest GCS, M (SD)a | 14.51 (1.07) | 13.85 (1.14) | 11.46 (1.95) | 5.70 (2.26) | |
| Duration of Coma, n (%)a | |||||
| None | 28 (53) | 3 (23) | 6 (27) | 1 (10) | |
| <5 min | 17 (32) | 8 (62) | 14 (64) | 4 (40) | |
| >5 min <24 h | 3 (6) | 0 (0) | 0 (0) | 1 (10) | |
| >24 h | 0 (0) | 0 (0) | 0 (0) | 4 (40) | |
| Unknown | 5 (9) | 2 (15) | 2 (9) | 0 (0) | |
| Surgical intervention, n (%) | 0 (0) | 0 (0) | 7 (32) | 6 (60) | |
| Cause of injury, n (%)a | |||||
| MVA (passenger, cyclist, pedestrian) | 3 (6) | 2 (15) | 9 (41) | 5 (50) | |
| Fall/Blow | 50 (94) | 11 (85) | 13 (59) | 5 (50) | |
aStatistically significant difference between groups, P < 0.05.
bSES is based on the ANZSCO. The scale ranges from 0 to 100 with higher scores reflecting higher occupational status of the primary caregiver.
For injury variables, comparisons of the severity groups showed expected differences: surgical intervention (all P < 0.001), lowest GCS (all P < 0.001) and duration of coma (P < 0.001). Cause of injury was predominately falls/blows for Mild (94%) and mild-complicated TBI (85%). For the Moderate and Severe TBI groups, falls/blows (59 and 50%, respectively) and motor vehicle accidents (41 and 50%, respectively) were equally common. The groups did not differ for age at injury.
The effect of pediatric TBI on global cortical morphology
Table 2 displays the group means and SDs for total cortical volume (TCV), cortical gray matter (GM), cortical white matter (WM), and CC volume. MANOVA for these measures of global cortical morphology was not significant, F(12, 320.427) = 1.527, P = 0.113.
Table 2.
The effect of TBI on global cortical volume
| Volume cm3 (SD) | Control | Mild | Mild-complex | Moderate TBI | Severe TBI | F | P-value | η2 |
|---|---|---|---|---|---|---|---|---|
| TCV | 714.82 (36.68) | 709.40 (44.80) | 734.49 (24.98) | 724.38 (45.96) | 719.80 (51.97) | 0.996 | 0.413 | 0.03 |
| Total CC | 2.04 (0.32) | 1.93 (0.31) | 2.09 (0.21) | 2.01 (.29) | 2.08 (0.25) | 1.566 | 0.188 | 0.04 |
| Total GM | 440.33 (25.81) | 442.01 (33.26) | 462.10 (26.35) | 452.35 (33.50) | 436.94 (65.49) | 1.255 | 0.291 | 0.04 |
| Total WM | 274.49 (20.54) | 267.39 (22.44) | 272.39 (20.86) | 272.02 (20.16) | 282.86 (33.58) | 2.181 | 0.075 | 0.06 |
TCV, total cortical volume; CC, corpus callosum; GM, gray matter; WM, white matter.
The impact of TBI on SBN morphology
ANCOVA covarying for ICV, age and SES revealed a significant main effect of injury severity on volume of the overall SBN package, F(4, 131) = 9.573, P < 0.001. As shown in Table 3, post hoc pairwise comparisons revealed that the severe TBI group showed significantly reduced SBN volume relative to TD controls (P < 0.001), and children with mild, mild complicated, and moderate TBI (all P < 0.001). The MANOVA for regions within the network was significant, F(40, 437.922) = 1.770, P = 0.003. Group differences in the overall SBN package and ROIs are presented in Table 3.
Table 3.
Group differences in volume of SBN and ROI
| Network/Region | Control | Mild | Mild-complex | Moderate TBI | Severe TBI | F | P-value | η2 |
|---|---|---|---|---|---|---|---|---|
| SBN | 189 559 | 194 590 | 190 039 | 191 081 | 171 038a,b,c,d | 9.573 | 0.000 | |
| STS | 23 049 | 23 519 | 22 698 | 23 029 | 20 741a,b,c,d | 2.603 | 0.039 | 0.08 |
| FG | 10 043 | 11 319 | 10 085 | 10 699 | 10 175b | 2.726 | 0.032 | 0.08 |
| TP | 11 842 | 12 172 | 11 851 | 12 671 | 10 250a,b,c,d | 4.206 | 0.003 | 0.12 |
| mPFC | 2010 | 2048 | 1776 | 1991 | 1639a,b,d | 2.669 | 0.035 | 0.08 |
| FP | 5010 | 5216 | 5334 | 5061 | 4683 | 1.054 | ns | 0.03 |
| OFC | 33 572 | 35 601 | 32 594e | 33 798f | 29 743a,b,c,d | 8.757 | 0.000 | 0.22 |
| TPJ | 38 044 | 38 319 | 38 231 | 37 302 | 34 401a,b,c,d | 3.081 | 0.019 | 0.09 |
| Cingulate | 35 635 | 36 620 | 34 903 | 35 531 | 31 695a,b,c,d | 6.163 | 0.000 | 0.17 |
| Insula | 26 500 | 27 385 | 26 456 | 26 961 | 25 504a,b,c,d | 2.300 | 0.062 | 0.07 |
| Amygdala | 3334 | 3507 | 3195 | 3422 | 3165b,d | 2.641 | 0.037 | 0.08 |
Note: statistically significant (P < 0.05) analyses comparing. aTD vs TBI-Severe; bTBI-Mild vs TBI-Severe; cTBI-Mild complex vs TBI-Severe; dTBI-Moderate vs TBI-Severe; eMild vs Mild Complex; fTBI-Mild vs TBI-Moderate.
STS, superior temporal sulcus; FG, fusiform gyrus; TP, temporal pole; mPFC, medial prefrontal cortex; FP, frontal pole; OFC, orbitofrontal cortex; TPJ, temporo-parietal junction.
The impact of TBI on ToM and behavior problems
TBI vs control
ANCOVA covarying for age at testing and SES revealed that the TBI and TD control groups differed significantly on ToM, F(1,123) = 4.77, P = 0.03, η2 = 0.04; and CBCL Total Problems, F(1,103) = 5.06, P = 0.03, η2 = 0.05. Analysis of the impact of group on ToM task type revealed that the TBI group performed significantly worse than TD controls on affective ToM [F(1,123) = 4.23, P = 0.04, η2 = 0.04] and conative ToM [F(1,123) = 4.11, P = 0.04, η2 = 0.04]. Children with TBI also showed worse performance than TD controls for cognitive ToM, but the difference was not significant, F(1,123) = 2.93, P = 0.090, η2 = 0.03.
Injury severity
The injury severity groups differed significantly on the composite, cognitive and conative ToM measures. As shown in Table 4, planned contrasts indicated that the severe TBI group performed significantly worse than the TD control group on the composite and cognitive ToM measures. Similarly, the mild complicated TBI group performed significantly worse than TD controls on composite and conative ToM measures. Moreover, the TD control group showed significantly better conative ToM than the moderate TBI group.
Table 4.
Group means and SDs for ToM and CBCL total behavior problems
| TD | Mild TBI | Mild-complex TBI | Moderate TBI | Severe TBI | ||||
|---|---|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | F | P-value | η2 | |
| ToM Composite | 74.19 (14.98) | 70.49 (12.88) | 65.22 (15.17)a | 68.20 (15.62) | 63.97 (20.66)b | 3.66 | 0.03 | 0.06 |
| Cognitive ToM | 80.14 (27.31) | 78.13 (22.26) | 78.93 (27.41) | 75.00 (27.67) | 61.11 (32.74)b | 3.20 | 0.04 | 0.05 |
| Conative ToM | 75.01 (29.41) | 72.92 (29.35) | 49.74 (35.13)a,c | 65.40 (28.10)d | 74.07 (37.33) | 2.51 | 0.04 | 0.08 |
| Affective ToM | 67.42 (15.23) | 60.43 (11.39) | 67.00 (17.74) | 64.20 (19.55) | 56.74 (14.81) | 1.48 | 0.21 | 0.05 |
| CBCL Total Raw | 12.03 (8.42) | 16.78 (16.46) | 25.22 (21.98) | 21.61 (25.38) | 27.00 (16.87) | 2.18 | 0.077 | 0.08 |
Note: significant (P < 0.05) analyses comparing; aTD vs TBI-Mild complex; bTD vs TBI-Severe; cTBI-Mild vs TBI-Mild Complex; dTD vs TBI-Moderate.
TBI, traumatic brain injury; TD, typically developing control; M, mean; SD, standard deviation; CBCL, child behavior checklist.
In the ANCOVA with CBCL Total Problems as the dependent variable, the omnibus test for group differences between severity groups was not significant (Table 4). However, the planned linear contrast between groups was significant, F(1, 102) = 5.87, P = 0.02. The mild complicated (t = 2.05, P = 0.04, d = 0.79) and severe TBI group (t = −2.22, P = 0.03, d = 1.12) showed significantly more behavior problems than the TD control group.
Prospective relations among social brain morphology, post-acute ToM and chronic behavior problems
Within the TBI group, more frequent behavior problems were significantly related to reduced volume of the overall SBN package, as well as the STS, and cingulate cortex (Table 5). Moreover, poorer ToM was significantly associated with reduced volume of the SBN, STS, mPFC, cingulate cortex, OFC and insula, as well as more frequent behavior problems.
Table 5.
Partial correlations between social brain morphology, ToM and behavioral problems, r(p)
| Network/Region | ToM | CBCL total problems |
|---|---|---|
| SBN | 0.325 (0.007)* | −0.275 (0.022)* |
| STS | 0.241 (0.048)* | −0.330 (0.006)* |
| FG | 0.120 (0.326) | −0.032 (0.795) |
| TP | −0.026 (0.834) | −0.100 (0.414) |
| mPFC | 0.273 (0.023)* | −0.221 (0.069) |
| FP | 0.008 (0.950) | 0.041 (0.735) |
| Cingulate cortex | 0.265 (0.028)* | −0.239 (0.048)* |
| OFC | 0.244 (0.043)* | −0.152 (0.212) |
| TPG | 0.198 (0.103) | −0.195 (0.108) |
| Insula cortex | 0.376 (0.001)* | −0.139 (0.256) |
| Amygdala | −0.005 (0.970) | 0.059 (0.630) |
| ToM | −0.377 (0.001)* |
*Significant partial correlation covarying for ICV and age at MRI.
Mediation analyses
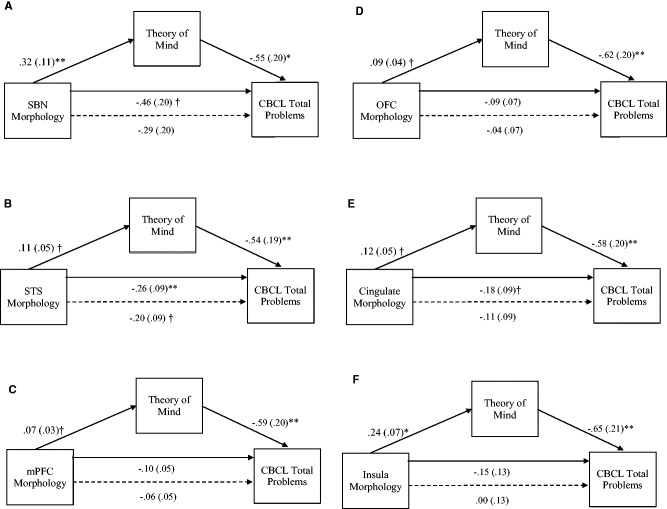
The results from the mediator models are presented in Figures 1A–E and Table 6. Overall SBN and five regional measures of SBN morphology were significantly related to ToM, and were entered into separate mediator models to test the hypothesis that ToM mediates the prospective relationship between SBN morphology and chronic behavior problems. As shown in Figure 1A–F, the effect of SBN, STS, mPFC, OFC, cingulate and insula were significant, indicating that reduced volume was associated with poorer ToM. In predicting behavior problems, SBN, STS and cingulate had a significant direct effect on CBCL Total Problems. mPFC, OFC and insula did not directly predict CBCL Total Problems.
Fig. 1.
(A-F) The effect of SBN morphology on CBCL Total Behavior Problems through ToM. When ToM was included in the model the direct effect of SBN morphology (dashed line) was no longer significant, indicating a significant indirect effect. Unstandardized path coefficients (SE) shown for each path. *P < 0.001; **P < 0.01, †P < 0.05.
Table 6.
Model summary information: indirect effects of social brain morphology on outcome
| Outcome: CBCL | Indirect effects |
||
|---|---|---|---|
| Effect | Lower CI | Upper CI | |
| SBN | |||
| ToM* | −0.175 | −0.440 | −0.034 |
| STS* | |||
| ToM* | −0.059 | −0.158 | −0.006 |
| mPFC | |||
| ToM* | −0.042 | −0.112 | −0.005 |
| OFC | |||
| ToM* | −0.054 | −0.145 | −0.007 |
| Cingulate | |||
| ToM* | −0.069 | −0.182 | −0.011 |
| Insula | |||
| ToM* | −0.153 | −0.352 | −0.043 |
CI, Confidence interval; CBCL, Child Behavior Check List
*significant indirect effect
ToM was a direct predictor of CBCL Total Problems in each model. Indirect effects of SBN morphology on CBCL Total Problems via the proposed mediator (i.e. theory of mind) are presented in Table 6. As shown in Table 6, the test of the indirect effect of brain morphology on outcome, when ToM was included in the models, was significant for SBN (Fig. 1A), STS (Fig. 1B), mPFC (Fig. 1C), OFC (Fig. 1D), cingulate (Fig. 1E) and insula (Fig. 1F).
Discussion
The primary aims of this study were to evaluate (i) the sub-acute effects of pediatric TBI on SBN morphology; and (ii) whether ToM mediates the prospective association between structural change in the SBN and more frequent chronic behavior problems. In keeping with expectations, severe paediatric TBI was associated with volumetric reductions in the overall SBN package and its putative hub regions at 2-months post-injury. Moreover, ToM mediated the relationship between abnormal SBN morphology and chronic behavior problems, indicating that sub-acute morphological changes in the SBN prospectively predict chronic behavioral symptoms through impaired ToM.
In this study, we show that severe TBI is associated with sub-acute volumetric reductions in the overall SBN package, together with regional GM morphological abnormalities of the STS, TPJ, fusiform, TP, mPFC, OFC, cingulate and amygdala. In contrast to the pattern of generalized structural brain changes previously documented in children with chronic severe TBI (Wilde et al., 2005; Spanos et al., 2007; Fearing et al., 2008; Merkley et al., 2008; Bigler et al., 2010; Wu et al., 2010a; Beauchamp et al., 2011a,b; Levin et al., 2011), no significant group differences were identified on measures of global cortical volume, including indices of total cortical gray, white and CC volume. Overall, regional volumetric reductions in the OFC, amygdala, cingulate and TP converge with previous evidence for the selective vulnerability of frontotemporolimbic regions to acceleration-deceleration forces of pediatric TBI (Wilde et al., 2005; Bigler et al., 2010, 2013; McAllister, 2011; Bigler, 2013), and suggest that selective structural changes in these regions may be detectable as early as 2-months post-severe TBI.
The selective pattern of sub-acute structural change among children with severe TBI likely reflects the initial neuropathological consequences of primary and secondary injury mechanisms, which interact dynamically in the pathogenesis of GM tissue loss. Since frontal and temporo-limbic regions of the SBN are highly susceptible to surface contusions, biomechanical shearing and compressive injuries, focal injuries to these regions likely cause brain tissue deformation and induce neuroinflammatory reactions that compromise cerebral perfusion, thereby affecting cellular degradation and apoptosis (Xu et al., 2010). Previous adult studies show that severe TBI is associated with widely distributed pathological effects throughout the brain, including dynamic patterns of primary-trauma induced cell loss that occur within hours of injury and continue beyond 2-years post-injury (Blatter et al., 1997; Adams et al., 2011; Bigler, 2013). For example, Blatter et al. examined longitudinal changes in ventricle-to-brain ratio (VBR) among adults with TBI and showed that while the steepest VBR increases occurred at ∼3-weeks post-injury, VBR continued to increase beyond 2-years after injury. Although the current findings converge with previous adult data to suggest that the initial neuropathological consequences of cellular phagocytosis may be apparent at 2-months post-injury, few studies have evaluated the prognostic significance of these sub-acute structural brain changes for post-injury social behavior.
In partial support of our hypotheses, we found evidence for post-acute theory of mind impairment among children with severe TBI, and children classified with mild complicated TBI on the basis of positive research MR findings. Although these findings are consistent with previous reports of social cognitive dysfunction after severe pediatric TBI (Chertkoff Walz et al., 2010; Tlustos et al., 2011; Dennis et al., 2013a,b,c; Ryan et al., 2014b), we provide preliminary evidence that theory of mind is vulnerable to the effects of milder generalized injuries in mid-late childhood and adolescence. These results converge to support the poor prognostic utility of clinical measures of injury severity (Dennis et al., 2012; Anderson et al., 2013), and suggest that clinicians should exercise caution when prognosticating based on early clinical indictors.
We also found that post-acute theory of mind impairment was associated with volumetric reductions in the overall SBN package at 2-months post-injury. Previous retrospective studies of chronic pediatric TBI have documented atrophy in large-scale functional networks including the default mode, salience and mirror-neuron empathy networks (Dennis et al., 2013c); however, the links between abnormal network morphology and impaired ToM have been inconsistent. For example, Dennis et al. (2013c) found that impaired ToM was related to atrophy in an overall package of functional networks, but no individual network reached statistical significance. Our prospective analysis extends these previous reports to suggest that sub-acute morphological changes in the SBN may be a useful imaging biomarker for prediction of post-acute ToM impairment.
Consistent with previous studies (Ryan et al., 2013, 2015a,c), our findings reveal a prospective association between post-acute ToM impairment and more frequent chronic behavior problems. Moreover, as predicted, our results reveal that ToM mediates the prospective relationship between abnormal SBN morphology and chronic behavior problems after pediatric TBI. The SBN is known to mediate a range of highly specialized social cognitive functions that continue to illustrate protracted development into mid-to-late adolescence (Choudhury et al., 2006; Blakemore, 2008; Dumontheil et al., 2010; Burnett et al., 2011; Kennedy and Adolphs, 2012). Given that successful real world functioning depends on these functions to maintain appropriate behavior and achieve developmentally appropriate social goals (Beauchamp and Anderson, 2010), it is perhaps not surprising that impaired ToM mediates the relationship between sub-acute structural change in the SBN and chronic behavior problems.
In keeping with expectations, morphological abnormalities of multiple cortical regions of the SBN- including the STS, OFC, mPFC, cingulate and insula indirectly contributed to more frequent chronic behavioral symptoms via their influence on post-acute ToM. Neuroanatomical and functional abnormalities of the STS and insula have been implicated in number of psychiatric disorders characterized by impaired social cognition, including autism (Boddaert et al., 2004; Zilbovicius et al., 2006; Uddin and Menon, 2009) and schizophrenia (Wylie and Tregellas, 2010; Shin et al., 2015). Moreover, an emerging body of evidence from adult populations suggests that the mPFC, cingulate and STS represent integrative cortical hub regions for various aspects of socially driven interactions, including social perception and empathy (Iacoboni et al., 2004; Walter et al., 2004; Frith and Frith, 2006; Singer et al., 2009; Bernhardt and Singer, 2012; Lahnakoski et al., 2012; Lavin et al., 2013); however, it remains unclear whether these social behaviors share similar neuroanatomical substrates in the developing social brain. In addressing the paucity of pediatric research in this area, we show that morphological abnormalities of these putative hub regions prospectively contribute to impaired ToM, which in turn elevates risk for chronic maladaptive behaviors after pediatric TBI. Since structural change in these regions represents a proxy for disruption to underlying SBN connectivity (Bigler, 2013; Spitz et al., 2013), our findings suggest that impaired ToM represents both a primary symptom of underlying network disruption, and a mechanism that links pediatric TBI to chronic behavior difficulties.
Our results suggest that MR based morphometry has the potential to unlock early prognostic biomarkers that can potentially add predictive value to traditional clinical indicators. In contrast to previous reports of a clear dose-response relationship between injury severity and ToM outcomes (Dennis et al., 2012; Ryan et al., 2014b), we found that post-acute ToM impairment was apparent among children with severe TBI, as well as children and adolescents with mild-complicated injury based on positive research MR findings. In addition, we showed that morphological abnormalities of the SBN are detectable at 2-months post-TBI, and may represent a useful imaging biomarker for early identification of children at elevated risk for ToM impairment at 6-months post-injury. Longer-term follow-up incorporating social cognitive assessment of these high-risk children may help identify those patients who are likely to benefit from targeted psycho-education and social-communicative interventions designed to reduce risk for maladaptive behavior trajectories.
The strength of the present findings is weakened by sole reliance on parent report for measuring behavior problems, as well as by a lack of information on pre-injury functioning. Moreover, reliance on a single imaging technique should prompt further investigation into potential relationships between measures of cortical volume and WM microstructure derived from diffusion tensor imaging and tractography. In particular, recent studies of adult TBI suggest that disrupted structural network connectivity is a key factor in TBI outcomes (Hillary et al., 2011; Wang et al., 2011; Caeyenberghs et al., 2012, 2014). On this basis, efforts to quantify alterations in structural network topology using graph theory methods may assist to improve prognostic accuracy, and provide a further window into the neuroanatomical and pathophysiological correlates of social cognitive and behavioral dysfunction after pediatric TBI.
Moreover, while the current findings converge to support the selective vulnerability of the SBN to the sub acute effects of pediatric TBI, the consequences of these morphological abnormalities for ongoing brain maturation are poorly understood. Although previous reports suggest that the effects of primary-trauma induced cell loss interact with a number of potential secondary pathological mechanisms to contribute to long-term, generalized neurodegenerative effects (Bigler, 2013), further longitudinal follow-up of these children across mid-to-late adolescence may assist to elucidate how the effects of TBI interact with ongoing processes of myelination and GM synaptic pruning during periods that coincide with rapid refinement and consolidation of social cognitive neural networks.
Conclusions
In one of the largest prospective studies to investigate the sub-acute impact of pediatric TBI on social brain network morphology, we demonstrate that severe pediatric TBI is associated with volumetric reductions in the overall SBN package and its putative hub regions at 2-months post-injury. In addressing the paucity of research examining the mechanisms that link pediatric TBI to long-term behavior problems, post-acute theory of mind impairment was found to mediate the prospective association between sub-acute structural change in the SBN and long-term behavior problems. More specifically, our results suggest that sub-acute volumetric changes in the SBN and/or its putative hub regions may represent valuable prognostic markers for early identification of children at elevated risk for post-acute social cognitive dysfunction, which may in turn contribute to chronic behavior problems.
Funding
This work was supported by a grant from the Victorian Neurotrauma Initiative (No. CO6E1), Australia; the Victorian Government Operational Infrastructure Support Program; an Australian Postgraduate Award, MCRI PhD scholarship, and National Health and Medical Research Council (NHMRC) Centre for Research Excellence in Brain Injury Recovery Seed Grant to N.R.; and an NHMRC Senior Practitioner Fellowship to V.A. The funding bodies did not play a role in the design of the study, collection, analysis and interpretation of the data, or writing of the article.
Conflict of interest. None declared.
References
- Achenbach T.M., Rescorla L. (2001). ASEBA School-Age Forms and Profiles. Burlington: Aseba. [Google Scholar]
- Adams J.H., Jennett B., Murray L.S., Teasdale G.M., Gennarelli T.A., Graham D.I. (2011). Neuropathological findings in disabled survivors of a head injury. Journal of Neurotrauma, 28(5), 701–9. [DOI] [PubMed] [Google Scholar]
- Adolphs R. (2009). The social brain: neural basis of social knowledge. Annual Review of Psychology, 60, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V., Beauchamp M.H., Yeates K.O., Crossley L., Hearps S.J., Catroppa C. (2013). Social competence at 6 months following childhood traumatic brain injury. Journal of the International Neuropsychological Society, 19(5), 539–50. [DOI] [PubMed] [Google Scholar]
- Baron R.M., Kenny D.A. (1986). The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51(6), 1173. [DOI] [PubMed] [Google Scholar]
- Beauchamp M., Ditchfield M., Maller J., et al. (2011a). Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury. International Journal of Developmental Neuroscience, 29(2), 137–43. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.H., Anderson V. (2010). SOCIAL: an integrative framework for the development of social skills. Psychological Bulletin, 136(1), 39. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.H., Ditchfield M., Catroppa C., et al. (2011b). Focal thinning of the posterior corpus callosum: normal variant or post-traumatic? Brain Injury, 25(10), 950–7. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Singer T. (2012). The neural basis of empathy. Annual Review of Neuroscience, 35, 1–23. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. (2013). Traumatic brain injury, neuroimaging, and neurodegeneration. Frontiers in Human Neuroscience, 7, 395, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler E.D., Abildskov T.J., Petrie J., et al. (2013). Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology, 27(4), 438–51. [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Abildskov T.J., Wilde E.A., et al. (2010). Diffuse damage in pediatric traumatic brain injury: a comparison of automated versus operator-controlled quantification methods. Neuroimage, 50(3), 1017–26. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–77. [DOI] [PubMed] [Google Scholar]
- Blatter D.D., Bigler E.D., Gale S.D., et al. (1997). MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. American Journal of Neuroradiology, 18(1), 1–10. [PMC free article] [PubMed] [Google Scholar]
- Boddaert N., Chabane N., Gervais H., et al. (2004). Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage, 23(1), 364–9. [DOI] [PubMed] [Google Scholar]
- Burnett S., Sebastian C., Kadosh K. C., Blakemore S.-J. (2011). The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience & Biobehavioral Reviews, 35(8), 1654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K., Leemans A., Heitger M.H., et al. (2012). Graph analysis of functional brain networks for cognitive control of action in traumatic brain injury. Brain, 135(4), 1293–307. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Leemans A., Leunissen I., et al. (2014). Altered structural networks and executive deficits in traumatic brain injury patients. Brain Structure and Function, 219(1), 193–209. [DOI] [PubMed] [Google Scholar]
- Chertkoff Walz N., Owen Yeates K., Gerry Taylor H., Stancin T., Wade S.L. (2010). Theory of mind skills 1 year after traumatic brain injury in 6 to 8 year old children. Journal of neuropsychology, 4(2), 181–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S., Blakemore S.-J., Charman T. (2006). Social cognitive development during adolescence. Social Cognitive and Affective Neuroscience, 1(3), 165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–94. [DOI] [PubMed] [Google Scholar]
- Davies R.C., Williams W., Hinder D., Burgess C.N., Mounce L.T. (2012). Self-reported traumatic brain injury and postconcussion symptoms in incarcerated youth. The Journal of Head Trauma Rehabilitation, 27(3), E21–7. [DOI] [PubMed] [Google Scholar]
- Dennis M., Agostino A., Taylor H.G., et al. (2013a). Emotional expression and socially modulated emotive communication in children with traumatic brain injury. Journal of the International Neuropsychological Society, 19(1), 34–43. [DOI] [PubMed] [Google Scholar]
- Dennis M., Simic N., Agostino A., et al. (2013b). Irony and empathy in children with traumatic brain injury. Journal of the International Neuropsychological Society, 19(3), 338–48. [DOI] [PubMed] [Google Scholar]
- Dennis M., Simic N., Bigler E.D., et al. (2013c). Cognitive, affective, and conative theory of mind (ToM) in children with traumatic brain injury. Developmental Cognitive Neuroscience, 5, 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M., Simic N., Gerry Taylor H., et al. (2012). Theory of mind in children with traumatic brain injury. Journal of the International Neuropsychological Society, 18(5), 908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I., Apperly I. A., Blakemore S.J. (2010). Online usage of theory of mind continues to develop in late adolescence. Developmental Science, 13(2), 331–8. [DOI] [PubMed] [Google Scholar]
- Fearing M.A., Bigler E.D., Wilde E.A., et al. (2008). Morphometric MRI findings in the thalamus and brainstem in children after moderate to severe traumatic brain injury. Journal of Child Neurology, 23(7), 729–37. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–55. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., et al. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2006). The neural basis of mentalizing. Neuron, 50(4), 531–4. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76(4), 408–20. [Google Scholar]
- Hayes A.F. (2013) Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press. [Google Scholar]
- Herbet G., Lafargue G., Bonnetblanc F., Moritz-Gasser S., Duffau H. (2013). Is the right frontal cortex really crucial in the mentalizing network? A longitudinal study in patients with a slow-growing lesion. Cortex, 49(10), 2711–27. [DOI] [PubMed] [Google Scholar]
- Hillary F., Slocomb J., Hills E., et al. (2011). Changes in resting connectivity during recovery from severe traumatic brain injury. International Journal of Psychophysiology, 82(1), 115–23. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Lieberman M.D., Knowlton B.J., Molnar-Szakacs I., Moritz M., Throop C.J., Fiske A.P. (2004). Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage, 21(3), 1167–73. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Griffin R., Csibra G., et al. (2005). The emergence of the social brain network: Evidence from typical and atypical development. Development and Psychopathology, 17(3), 599–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J., Czanner S., Greve D., et al. (2006). Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage, 30(2), 436–43. [DOI] [PubMed] [Google Scholar]
- Kennedy D.P., Adolphs R. (2012). The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences, 16(11), 559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny D.T., Lennings C.J. (2007). Relationship between head injury and violent offending in juvenile detainees. NSW Crime and Justice Bulletin, 107, 1–15. [Google Scholar]
- Lahnakoski J.M., Glerean E., Salmi J., et al. (2012). Naturalistic FMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Frontiers in Human Neuroscience, 6, 233, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin C., Melis C., Mikulan E., Gelormini C., Huepe D., Ibañez A. (2013). The anterior cingulate cortex: an integrative hub for human socially-driven interactions. Frontiers in Neuroscience, 7, 64, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H.S., Wilde E.A., Hanten G., et al. (2011). Mental state attributions and diffusion tensor imaging after traumatic brain injury in children. Developmental Neuropsychology, 36(3), 273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Liu J. (2013). The effect of pediatric traumatic brain injury on behavioral outcomes: a systematic review. Developmental Medicine and Child Neurology, 55(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max J.E., Wilde E.A., Bigler E.D., et al. (2012). Neuroimaging correlates of novel psychiatric disorders after pediatric traumatic brain injury. Journal of the American Academy of Child and Adolescent Psychiatry, 51(11), 1208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T. W. (2011). Neurobiological consequences of traumatic brain injury. Dialogues in Clinical Neuroscience, 13(3), 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A., Corrigan J., Horwood L., Fergusson D. (2013). Substance abuse and criminal activities following traumatic brain injury in childhood, adolescence, and early adulthood. The Journal of Head Trauma Rehabilitation , 29(6), 498–506. [DOI] [PubMed] [Google Scholar]
- McKinlay A., Grace R., Horwood L., et al. (2008). Prevalence of traumatic brain injury among children, adolescents and young adults: prospective evidence from a birth cohort. Brain Injury, 22(2), 175–81. [DOI] [PubMed] [Google Scholar]
- McMillan J., Beavis A., Jones F.L. (2009). The AUSEI06 A new socioeconomic index for Australia. Journal of Sociology, 45(2), 123–49. [Google Scholar]
- Merkley T.L., Bigler E.D., Wilde E.A., McCauley S.R., Hunter J.V., Levin H.S. (2008). Short communication: Diffuse changes in cortical thickness in pediatric moderate-to-severe traumatic brain injury. Journal of Neurotrauma, 25(11), 1343–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E., Indig D., Haysom L. (2014). Traumatic brain injury, mental health, substance use, and offending among incarcerated young people. The Journal of Head Trauma Rehabilitation, 29(3), 239–47. [DOI] [PubMed] [Google Scholar]
- Robinson K.E., Fountain-Zaragoza S., Dennis M., et al. (2014). Executive functions and theory of mind as predictors of social adjustment in childhood traumatic brain injury. Journal of Neurotrauma, 31(22), 1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan N.P., Anderson V., Godfrey C., et al. (2014a). Predictors of very-long-term sociocognitive function after pediatric traumatic brain injury: evidence for the vulnerability of the immature “social brain”. Journal of Neurotrauma, 31(7), 649–57. [DOI] [PubMed] [Google Scholar]
- Ryan N.P., Anderson V., Godfrey C., Eren S., Rosema S., Taylor K., Catroppa C. (2013). Social communication mediates the relationship between emotion perception and externalizing behaviors in young adult survivors of pediatric traumatic brain injury (TBI). International Journal of Developmental Neuroscience, 31(8), 811–9. [DOI] [PubMed] [Google Scholar]
- Ryan N.P., Catroppa C., Beare R., Coleman L., et al. (2015a). Predictors of longitudinal outcome and recovery of pragmatic language and its relation to externalizing behaviour after pediatric traumatic brain injury. Brain and Language, 142, 86–95. [DOI] [PubMed] [Google Scholar]
- Ryan N.P., Catroppa C., Cooper J. M., et al. (2014b). Relationships between acute imaging Biomarkers and theory of mind impairment in post-acute pediatric traumatic brain injury: a prospective analysis using susceptibility weighted imaging (SWI). Neuropsychologia, 66, 32–8. [DOI] [PubMed] [Google Scholar]
- Ryan N.P., Catroppa C., Cooper J. M., et al. (2015b). The emergence of age dependent social cognitive deficits after generalized insult to the developing brain: a longitudinal prospective analysis using susceptibility-weighted imaging. Human Brain Mapping, 36(5), 1677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan N.P., Hughes N., Godfrey C., Rosema S., Catroppa C., Anderson V. A. (2015c). Prevalence and predictors of externalizing behavior in young adult survivors of pediatric traumatic brain injury. The Journal of Head Trauma Rehabilitation, 30(2), 75–85. [DOI] [PubMed] [Google Scholar]
- Shin J. E., Choi S.-H., Lee H., Shin Y. S., Jang D.-P., Kim J.-J. (2015). Involvement of the dorsolateral prefrontal cortex and superior temporal sulcus in impaired social perception in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 58, 81–8. [DOI] [PubMed] [Google Scholar]
- Singer T., Critchley H.D., Preuschoff K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences, 13(8), 334–40. [DOI] [PubMed] [Google Scholar]
- Sodian B. (2011). Theory of mind in infancy. Child Development Perspectives, 5(1), 39–43. [Google Scholar]
- Spanos G., Wilde E., Bigler E., et al. (2007). Cerebellar atrophy after moderate-to-severe pediatric traumatic brain injury. American Journal of Neuroradiology, 28(3), 537–42. [PMC free article] [PubMed] [Google Scholar]
- Spitz G., Bigler E.D., Abildskov T., Maller J.J., O’Sullivan R., Ponsford J.L. (2013). Regional cortical volume and cognitive functioning following traumatic brain injury. Brain and cognition, 83(1), 34–44. [DOI] [PubMed] [Google Scholar]
- Surian L., Caldi S., Sperber D. (2007). Attribution of beliefs by 13-month-old infants. Psychological Science, 18(7), 580–586. [DOI] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. (1974). Assessment of coma and impaired consciousness. Lancet, 2, 81–4. [DOI] [PubMed] [Google Scholar]
- Timonen M., Miettunen J., Hakko H., Zitting P., et al. (2002). The association of preceding traumatic brain injury with mental disorders, alcoholism and criminality: the Northern Finland 1966 Birth Cohort Study. Psychiatry Research, 113(3), 217–26. [DOI] [PubMed] [Google Scholar]
- Tlustos S.J., Chiu C.-Y.P., Walz N.C., Taylor H.G., Yeates K.O., Wade S.L. (2011). Emotion labeling and socio-emotional outcomes 18 months after early childhood traumatic brain injury. Journal of the International Neuropsychological Society, 17(6), 1132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Menon V. (2009). The anterior insula in autism: under-connected and under-examined. Neuroscience and Biobehavioral Reviews, 33(8), 1198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa R.A., Grados M., Slomine B., Herskovits E. H., Thompson R. E., Salorio C., Christensen J., Wursta C., Riddle M. A., Gerring J. P. (2004). Neuroimaging correlates of anxiety after pediatric traumatic brain injury. Biological psychiatry, 55(3), 208–16. [DOI] [PubMed] [Google Scholar]
- Walter H., Adenzato M., Ciaramidaro A., Enrici I., Pia L., Bara B.G. (2004). Understanding intentions in social interaction: the role of the anterior paracingulate cortex. Journal of Cognitive Neuroscience, 16(10), 1854–63. [DOI] [PubMed] [Google Scholar]
- Wang J., Bakhadirov K., Abdi H., et al. (2011). Longitudinal changes of structural connectivity in traumatic axonal injury. Neurology, 77(9), 818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M.A., de la Plata C.M., Spence J., et al. (2010). Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. Journal of Neurotrauma, 27(12), 2121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman H.M., Cross D., Watson J. (2001). Meta analysis of theory of mind development: the truth about false belief. Child Development, 72(3), 655–84. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Ayoub K.W., Bigler E.D., et al. (2012). Diffusion tensor imaging in moderate-to-severe pediatric traumatic brain injury: changes within an 18 month post-injury interval. Brain imaging and Behavior, 6(3), 404–16. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Chu Z., Bigler E.D., et al. (2006). Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma, 23(10), 1412–26. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Hunter J. V., Newsome M.R., et al. (2005). Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. Journal of Neurotrauma, 22(3), 333–44. [DOI] [PubMed] [Google Scholar]
- Williams W.H., Mewse A.J., Tonks J., Mills S., Burgess C. N., Cordan G. (2010). Traumatic brain injury in a prison population: prevalence and risk for re-offending. Brain Injury, 24(10), 1184–8. [DOI] [PubMed] [Google Scholar]
- Wu T.C., Wilde E.A., Bigler E.D., et al. (2010a). Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Developmental Neuroscience, 32(5–6), 361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.C., Wilde E.A., Bigler E.D., et al. (2010b). Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. Journal of Neurotrauma, 27(2), 303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie K.P., Tregellas J.R. (2010). The role of the insula in schizophrenia. Schizophrenia Research, 123(2), 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., McArthur D.L., Alger J.R., et al. (2010). Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism, 30(4), 883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates K.O., Swift E., Taylor H.G., et al. (2004). Short- and long-term social outcomes following pediatric traumatic brain injury. Journal of International Neuropsychological Society, 10(3), 412–26. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M., Meresse I., Chabane N., Brunelle F., Samson Y., Boddaert N. (2006). Autism, the superior temporal sulcus and social perception. Trends in Neurosciences, 29(7), 359–66. [DOI] [PubMed] [Google Scholar]