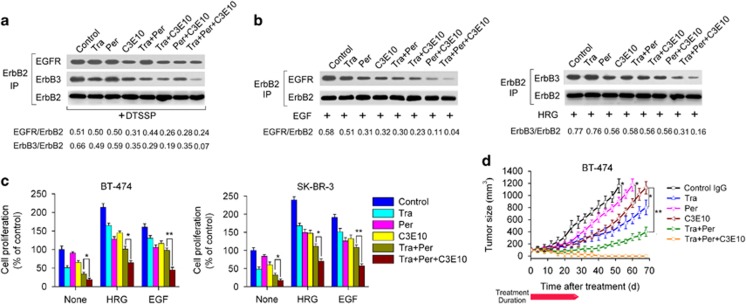
Figure 4.
The combination of trastuzumab, pertuzumab and C3E10 potently blocks ErbB2 heterodimerization and inhibits breast cancer cell growth. (a) Coimmunoprecipitation assay examining the ability of 100 nM of control IgG, trastuzumab, pertuzumab, C3E10, trastuzumab plus pertuzumab, trastuzumab plus C3E10, pertuzumab plus C3E10 or trastuzumab plus pertuzumab plus C3E10 to disrupt the ligand-independent association of ErbB2 with EGFR or ErbB3 in BT-474 cells. (b) Coimmunoprecipitation assay assessing the effects of 100 nM of control IgG, trastuzumab, pertuzumab, C3E10, trastuzumab plus pertuzumab, trastuzumab plus C3E10, pertuzumab plus C3E10 or trastuzumab plus pertuzumab plus C3E10 pretreatment on EGF-induced ErbB2/EGFR and HRG-induced ErbB2/ErbB3 heterodimerization in BT-474 cells. (c) MTS assay evaluating the effects of recombinant anti-ErbB2 mAbs on breast cancer cell proliferation in the absence or presence of ErbB ligand (EGF or HRG). Cells were incubated with 100 nM of control IgG, trastuzumab, pertuzumab, C3E10, trastuzumab plus pertuzumab or trastuzumab plus pertuzumab plus C3E10 for 2 h, followed by the addition of ErbB ligands or not. Recombinant human EGF and HRG were added at a final concentration of 5 and 1 nM, respectively. After an additional 4-day incubation, cell proliferation was determined by MTS assay. Results are shown as percentage of control cell proliferation. Error bars, s.d. *P<0.05; **P<0.001. (d) Tumor volume of BT-474 breast tumor xenografts after treatment with control IgG (5 mg/kg), trastuzumab (5 mg/kg), pertuzumab (5 mg/kg), C3E10 (5 mg/kg), trastuzumab plus pertuzumab (5 mg/kg each) or trastuzumab plus pertuzumab plus C3E10 (5 mg/kg each). Treatments consisted of twice weekly intravenous injection of different anti-ErbB2 mAbs for four consecutive weeks. Data are shown as means±s.e.m. *P<0.05; **P<0.001; Mann–Whitney test.