Figure 10.
Vacuolar Degradation of BRI1 Is Defective in alix-1 Mutants.
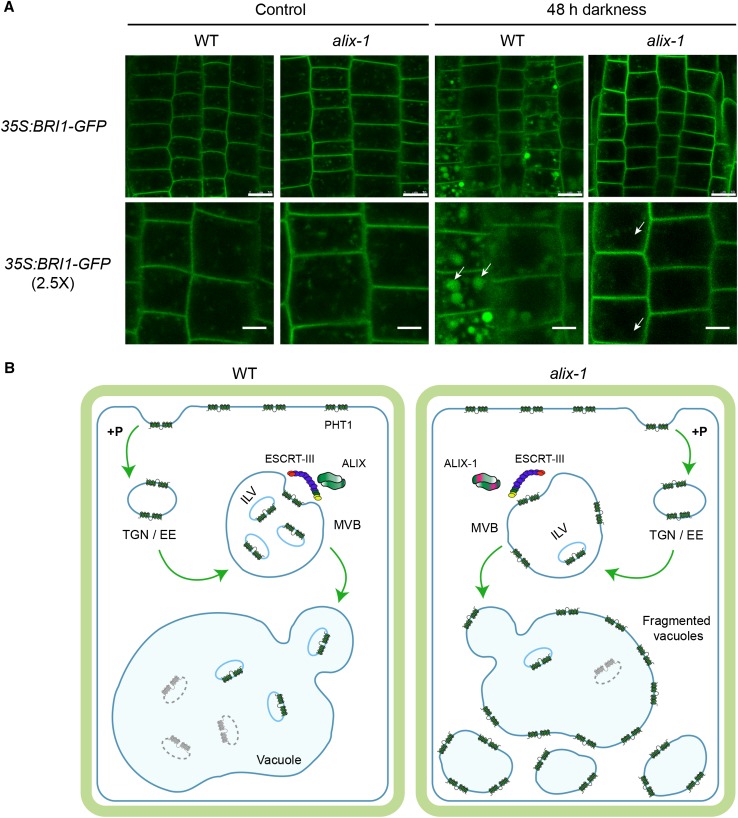
(A) Root epidermal cells from 5-d-old seedlings overexpressing BRI1-GFP in wild-type (WT) and alix-1 backgrounds grown in Pi-rich conditions were kept in the dark or in light for 48 h before they were observed by confocal imaging. White arrows point to vacuoles. Bars = 10 μm except in 2.5× zoom images, where bars = 5 μm.
(B) Proposed model for defects in PHT1 localization in alix-1 mutants. Under Pi-rich conditions (+P), Pi transporters (PHT1) are readily endocytosed at the plasma membrane and transported to the TGN/EE. TGN/EE vesicles mature to MVBs, where cargo proteins are packaged into ILVs by the action of ESCRT complexes and associated proteins (i.e., ALIX). Finally, MVBs fuse with vacuoles and release ILVs to the vacuole lumen where they are degraded together with their cargoes. In alix-1 mutants, ALIX-1 association with ESCRT-III complexes is altered, and PHT1;1 proteins are not correctly internalized into ILVs, being retained in the tonoplast upon MVB fusion with vacuoles. This effect leads to reduced PHT1 degradation. Defects in cargo trafficking in alix-1 also provoke vacuolar fragmentation, which alters Pi storage and subcellular distribution, and, therefore, Pi homeostasis.