Figure 4.
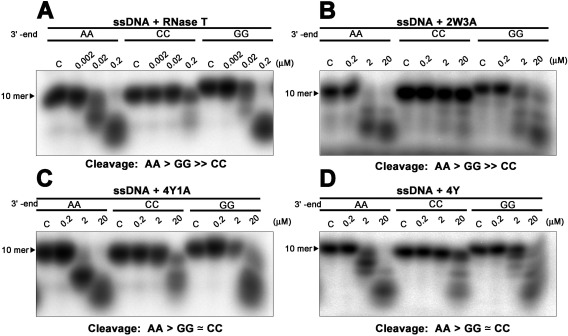
Mutating phenylalanine residues alters the cleavage preferences of RNase T. (A) RNase T was incubated with a single‐stranded DNA with a different sequence at 3'‐end: 5'‐AGTTATGAXX−3', XX = AA, GG, or CC. The exonuclease activity of the wild‐type RNase T is inhibited by the single‐stranded DNA with a 3'‐end CC, with a cleavage order of preference of AA > GG ≫ CC. (B) The RNase T mutant 2W3A (F29W/F77W/E73A/F124A/F146A) had weaker exonuclease activity but a similar sequence‐specific exonuclease activity to wild‐type RNase T. (C,D) The 4Y (F29Y/F77Y/F124Y/F146Y) and 4Y1A (F29Y/F77Y/F124Y/F146Y/E73A) mutants had an altered sequence‐dependent exonuclease activity compared to wild‐type RNase T with a cleavage preference of A > G ≃ C.
