Figure 3.
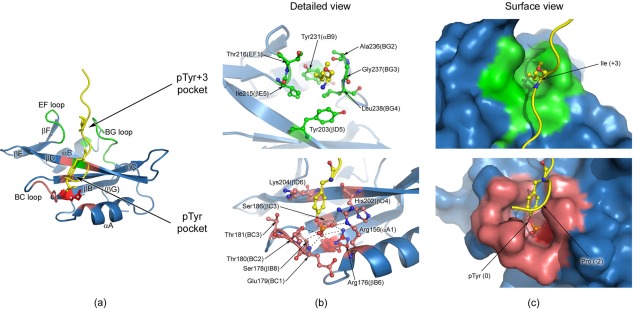
Human Fyn SH2:specific‐pY complex and detailed views of the two binding pockets. (a) The structure of the complex, with the regions forming the pTyr binding pocket colored in light red and the specificity pocket shown in green. The highly conserved Arg 176 (βB6) is depicted in red and represented in sticks. The specific‐pY peptide is colored in yellow and only the pTyr (0) and Ile (+3) residues are shown in sticks. (b) Detailed view of the two peptide binding pockets, where the main residues involved in interactions are represented in sticks. For the phosphate moiety the schematic directions of interaction are indicated by the dashed lines (bottom figure). Nitrogen atoms are coloured blue, oxygen atoms red, phosphorus atoms orange, and carbon atoms from the specific‐pY peptide yellow. The carbon atoms of the residues involved in the specificity pocket are shown in green (upper figure), while the ones from the pTyr pocket are depicted in light red (bottom figure). All the residues not directly involved in binding are coloured blue. The highly conserved Arg 176 (βB6) is colored red (bottom figure). (c) Surface view of the two pockets, where SH2 domain is shown in surface representation and the peptide is depicted as cartoon. The peptide interaction residues (pTyr and Ile+3) are illustrated as sticks. The structures in (b) and (c) have the same orientation.
