Figure 4.
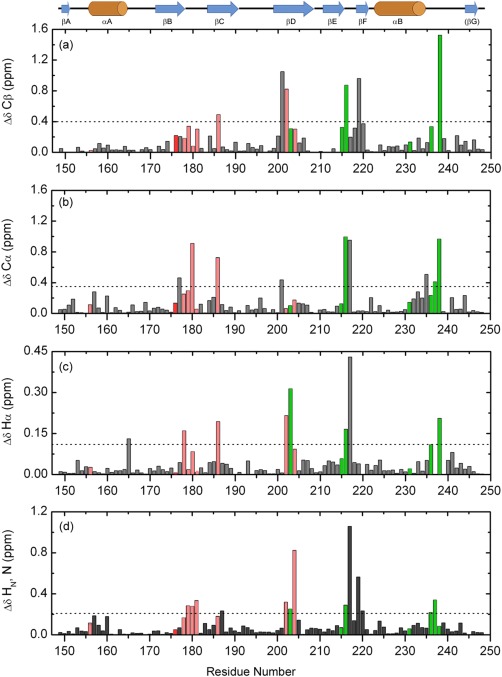
Chemical shift perturbations in Fyn SH2 domain upon specific‐pY peptide binding, in function of the residue number. The figure shows the absolute differences calculated for Cβ (a), Cα (b), Hα (c), and the combined backbone amides (d). The significance criteria are represented by the average chemical shift difference plus one standard deviation for each atom type, indicated by the dashed lines. The secondary structure elements of Fyn SH2 domain are depicted above. The residues involved in the specificity pocket are shown in green, while the ones from the pTyr pocket are depicted in light red. All the residues not directly involved in binding are colored gray. The highly conserved Arg 176 (βB6) is colored red.
