Figure 1.
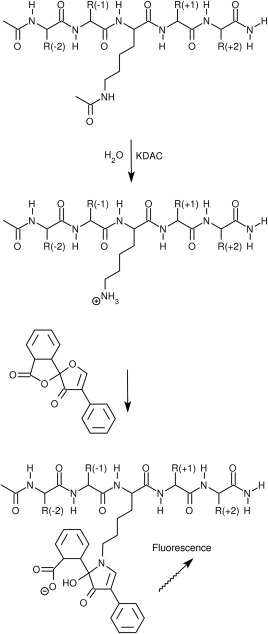
Fluorescamine reaction scheme. Peptide substrates, shown here as a 5‐mer, are initially deacetylated by a KDAC. After stopping the reaction, fluorescamine is added to generate a fluorescent product. Fluorescamine reacts more efficiently with primary amines than with secondary or tertiary amines, and only the product of a reaction with a primary amine is fluorescent. The N‐terminus and C‐terminus of the peptide are acetylated and amidated, respectively, to better mimic the presentation of a sequence within a longer protein sequence.
