Figure 2.
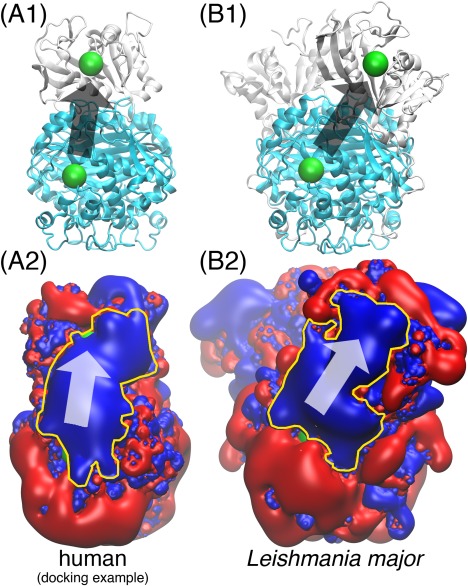
The human DHFR‐TS “electrostatic highway”. In (A1) and (B1), the cyan domain is the TS dimer; the white domain is DHFR (one monomer in (A1), two monomers in (B1)); the green spheres mark the location of the enzyme active sites. (A2) and (B2) display the electrostatics of the corresponding proteins in (A1) and (B1) with red and blue being the and electrostatic potential iso‐surfaces; The yellow contours highlight the “electrostatic highways.” The arrows in all four subfigures indicate the direction of substrate channeling. All of the proteins are displayed 90 degrees sideways compared to the front view in Fig. 1. The human DHFR and TS structures are the same as those used in Fig. 1; the Leishmania major DHFR‐TS structure is taken from Ref. 19. The docked human DHFR‐TS poses are capable of forming “electrostatic highways” for substrate channeling in a similar way to the Leishmania major DHFR‐TS, which is a well‐demonstrated case.
